1689
Neurofunctional topography of the human hippocampus: Converging data from a series of studies1Psychology, Auburn University, Auburn, AL, United States, 2Psychology, Auburn University, 3Siemens Healthcare, 4Electrical and Computer Engineering, Auburn University
Synopsis
The hippocampus, one of the most phylogenetically preserved structures in the human brain, has been a topic of interest to evolution theorists and cognitive neuroscientists alike. Malfunctions in the hippocampus are hallmark features in a number of psychiatric and neurological conditions, further amplifying interest. Little advancement has been made regarding the neurofunctional topography of the structure. We used high-field, high-resolution neuroimaging techniques to demonstrate convergent evidence on a new theory of hippocampal organization. Data from this project are expected to catalyze efforts in neuroscience and medicine to better understand hippocampal functioning and promote the identification of healthy and aberrant signaling.
INTRODUCTION
Arguably one of the most preserved neural structures across species, the hippocampus has been a prime target for cognitive neuroscientists. Remarkably, functional differentiation within the hippocampal formation has been posited in nearly all species over the past half century, with theories ranging from hemispheric specialization to more intricate models of topographical/subfield specialization. Unfortunately, the support for these hypothesized compartmentalizations, especially in the human functional neuroimaging literature, has been limited in scope due to the methodological approaches employed (e.g., lesion [case] studies, or single paradigm designs which have limited generalizability). As such, we propose a multifaceted, objective approach to identify neurofunctional differentiation within the hippocampus, leveraging the superior imaging capabilities of ultra high field, high resolution functional magnetic resonance imaging (fMRI). We believe that this approach could lead to transformative knowledge about the role of the human hippocampus in various neurocognitive processes, while also providing evidence for biosignatures indicative of healthy hippocampal function.METHODS
This study used a combination of ultra high field, high-resolution structural (i.e., diffusion tensor imaging [DTI]), and functional neuroimaging (i.e., functional magnetic resonance imaging [fMRI]) to discern the neurofunctional topography of the human hippocampus. Imaging data were acquired on a Siemens 7T MAGNETOM at the Auburn University MRI Research Center, using the following scanning parameters: fMRI (37 slices acquired parallel to the AC-PC line, 0.85mmx0.85mmx1.5mm voxels, TR/TE: 3000/28ms, 70° flip angle, base/phase resolution 234/100%, A>P phase encode direction, iPAT GRAPPA acceleration factor = 3, interleaved acquisition, 100 time points, total acquisition time 5:00); DTI (40 slices, 2mm3 isotropic voxels, TR/TE: 5200/94ms, base/phase resolution 122/100%, GRAPPA acceleration factor of 3, b = 0 and 1000, 30 directions, 3 averages, collected in an interleaved fashion, acquisition time = 8:21). Participants engaged in a resting state scan, as well as an encoding/recognition task in which they were asked to remember faces, scenes, and words. Clustering methods applied to the data included hierarchical clustering using an average-linking method, density peak clustering (DPC), and ordering points to identify the clustering structure (OPTICS) algorithm, described in previous publications1-4.RESULTS
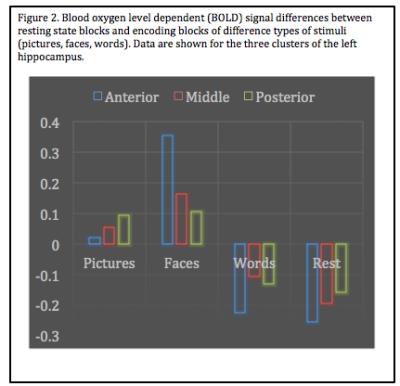
Data revealed a distinct neurofunctional topographical organization with nearly identical clustering during functional tasks for the left hippocampus, consistent with our previous research using robust meta-analytic connectivity modeling (MACM) 5 and coactivation based paracellation (CBP)6,7. Furthermore, patterns in the right hippocampus also showed stable, convergent topographical evidence (Figure 1). Interestingly, resting state data clustered more in line with neuroanatomical/cytoarchitectonic atlases, suggesting a possible dorsal-ventral explanation for evolutionary preservation. All task-based analyses identified an anterior-posterior segmentation. Examining the difference in encoding contexts, we also note that specific segmentations are associated with different types of stimuli in line with our previous CBP research6, providing preliminary evidence for topographical specification (Figure 2). Interestingly, the left hippocampus demonstrated greater convergence across methods compared to the right, suggesting that the right hippocampus may have hemisphere-specific functionality.DISCUSSION
These data, when combined with data published previously from our lab, demonstrate strong convergent evidence for the following: 1) hemispheric differences between the left and right hippocampus, 2) anterior-posterior neurofunctional topography during active recruitment of the hippocampus (i.e., tasks that engage the hippocampus), and 3) evidence for dorsal-ventral parcellation, consistent with neuroanatomical architecture, during a task independent states.CONCLUSION
Increasing our understanding of the neurofunctional role of the hippocampus could lead to theoretical advancements across disciplines, in addition to advancing our understanding of neurological and psychiatric disorders for which aberrant hippocampal functioning is a hallmark feature (e.g., posttraumatic stress disorder, medial temporal lobe epilepsy, depression, schizophrenia), providing an avenue for new clinical prevention and intervention strategies.Acknowledgements
No acknowledgement found.References
1 Ankerst, M., Breunig, M. M., Kriegel, H. P. & Sander, J. in SIGMOD 1999, Proceedings ACM SIGMOD International Conference on Management of Data, June 1–3, 1999 (eds A. Delis, C. Faloutsos, & S. Ghandeharizadeh) (ACM Press, 1999).
2 Dasgupta, S. & Long, P. Performance guarantees for hierarchical clustering. Journal of Computer and System Sciences 70, 555-569 (2005).
3 Liao, W., Chen, H., Yang, Q. & Lei, X. Analysis of fMRI data using improved self-organizing mapping and spatio-temporal metric hierarchical clustering. IEEE Trans on Medical Imaging 27, 1472-1483 (2008).
4 Rodriguez, A. & Laio, A. Clustering by fast search and find of density peaks. Science 344, 1492-1496, doi:10.1126/science.1242072 (2014).
5 Robinson, J. L., Laird, A. R., Glahn, D. C., Lovallo, W. R. & Fox, P. T. Metaanalytic connectivity modeling: Delineating the functional connectivity of the human amygdala. Human Brain Mapping 31, 173-184 (2010).
6 Robinson, J. L. et al. Neurofunctional topography of the human hippocampus. Human Brain Mapping 36, 5018-5037, doi:10.1002/hbm.22987 (2015).
7 Robinson, J. L., Salibi, N. & Deshpande, G. Functional connectivity of the left and right hippocampi: Evidence for functional lateralization along the long-axis using meta-analytic approaches and ultra-high field functional neuroimaging. NeuroImage 135, 64-78, doi:http://dx.doi.org/10.1016/j.neuroimage.2016.04.022 (2016).
Figures