Han Zhang1 and Dinggang Shen1,2
1Department of Radiology and BRIC, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 2Department of Brain and Cognitive Engineering, Korea University, Seoul, Korea, Republic of
Synopsis
Brain plasticity is fascinating and
important to our life. Radiation therapy can cause brain injury which may cover
or progress, posing an ideal case to study brain plasticity. We used a rare and
unique cohort of nasopharyngeal carcinoma patients with normal-appearing brains
to study irradiation injury in its preclinical stage in context of brain
functional and structural plasticity. We found an acute increase in local brain
activity, followed by its extensive reduction; and significant functional
connectivity loss in the default mode network. Such radiosensitive functional
alterations were intriguingly found to be plastic. By contrast, a progressive late
disrupted integrity of the related white matter was starting to be significant
after one year at the far end. Early increased local brain functional activity was
able to predict severe later brain necrosis through a bridge of brain
connectome. These findings highlight the importance of brain connectomics in
translational clinical study.
Introduction
RT is a longstanding and
routine clinical treatment for cancer. During cranial RT, normal brain tissue
along the RT pathway and in proximity to tumours is inevitably irradiated, causing
not only transient and reversible
abnormalities but also progressive
and irreversible late toxicities such as brain tissue necrosis. Preclinical detection of the irradiation injury, when no MR-visible lesions are developed,
is of great importance in the early prevention or mitigation of these
complications. The major constraints
of such studies have been: 1) a short survival time for brain tumour patients
that leaves us a limited follow-up time to investigate late responses, and 2) residual brain
lesions or recurrences that may not be easily differentiated from RT-induced injuries. Nasopharyngeal
carcinoma patients constitute an ideal cohort for such a study and can avoid the aforementioned problems. This study is to explore RT-induced brain injuries in
the preclinical stage in a voxel-wise
manner and to characterize their evolutional process. Materials and Methods
87 patients were involved (44.22
9.85 years; range: 19-68
years; 23 females). For details, please see Table 1. Sixty-two patients had been treated with RT. Based on the time between RT completion and MRI acquisition, subjects
were divided into four groups (G1, G2, G3, and G4; N = 25, 24, 19 and 19, respectively). We collected two non-invasive imaging modalities, rs-fMRI and DTI for each patients. We first conducted comparisons on local brain activity using fALFF maps. We then compared whole brain long-range co-activity using FC analysis with the seed defined at the PCC. Next, white matter microstructural integrity were compared using FA Analysis. The comparisons were carried out between post-RT groups (G2, G3, and G4) and the pre-RT
group (G1) using separate two-sample t-tests. Regarding the relationship between post-RT brain functional and
structural changes, we tested the hypothesis that changes in local brain activity
could occur with or be followed by a disrupted FC and/or reduction of white
matter microstructural integrity using DTI tractography. For patients in G2, G3 and G4, we conducted follow-up observations with
clinical MRI. Based on whether or not temporal lobe necrosis
happened in next five years after RT completion, we can retrospectively inspect
the earlier brain functional/structural changes in the preclinical stage to
search for any predictive biomarker or early sign of severe RT-injury.Result
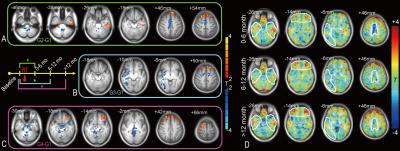
Compared with the baseline pre-RT group (G1), subjects in G2 (0 to 6 months
after RT) had increased fALFF in the left inferior temporal areas. G2-G1 also showed a fALFF increase in
the superior frontal area and a fALFF decrease in the supplementary motor area. G3-G1 showed a significant extensive
decrease in the fALFF in the dispersed right temporal area and part of the
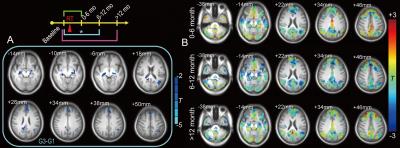
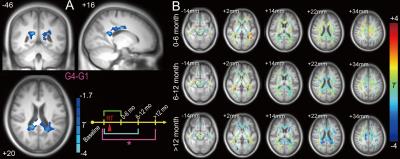
supplementary motor area (Fig. 1). There were no significant differences for G2-G1 and G4-G1. However, G3-G1 revealed a reduced FC with the PCC at various DMN regions (Fig. 2). Within an FA mask in putative white matter, there was a significant reduction in the FA
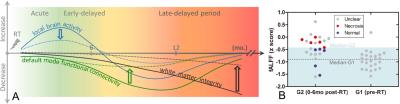
value in G4-G1 (Fig. 4A) but not in G3-G1 or G2-G1 (Fig. 3). Based on the above statistical analysis results, the schematic recovery/progression
course could be plotted and we derived a hypothesis to prevent or delay late effects, which is
called “preventive model” (Fig. 4). We also found fibers that connect between earlier functional
and later structural changes. There was a significant correlation between the PCC-to-right hippocampus
FC and the maximum dose applied to the right temporal lobe (r =
0.66, p < 0.05). The higher the maximum dose,
the lower the FC strength would be. Conclusion
By using a precious multimodal brain
imaging data, we revealed how the post-radiotherapy brain undergoes, in
preclinical stage, an evolutional process with both functional and structural
changes as well as both plastic recovery and progression. We have proposed a
dynamic process in the post-RT brain with earlier
vasculopathology dominance and later demyelination
dominance. Our results suggest both progressive
white matter degeneration from near- to far-end and neuroprotective processes and compensatory effects involve in
this recovery/progression course. Our preventive model highlights the
importance of preclinical brain function monitoring, careful RT-dose tailoring
and timely preventive neuroprotection for avoiding irreversible severe complications
that worsen outcomes.Acknowledgements
No acknowledgement found.References
No reference found.