1679
Functional and Metabolic Connectivity of the Awake and Anesthetized Brain1Radiology, University of Wisconsin, MADISON, WI, United States, 2Neuroscience, University of Wisconsin, MADISON, WI, United States, 3Psychology, University of Wisconsin, MADISON, WI, United States, 4Psychiatry, University of Wisconsin, MADISON, WI, United States
Synopsis
This study evaluated the effect of anesthesia on functional and metabolic brain connectivity, measured with simultaneous PET/MR using an awake monkey model. Results show a profound disruption of functional connectivity, effective connectivity, and novel approaches to metabolic connectivity following the administration of IV ketamine.
Purpose
This study evaluated the effect of anesthesia on functional and metabolic brain connectivity, measured with simultaneous PET/MR. Since the initial reports1, brain connectivity has been widely studied using fMRI2, and numerous networks can be identified where correlated signal fluctuations map to functionally specific anatomic regions. Recent availability of hybrid PET/MR scanners has extended the capability of these measurements, where PET agents specific to metabolism or neurotransmitter release can be measured in synchronicity with fMRI. Early studies, while equivocal, have suggested correlation of fMRI with 18F-FDG measurements of brain metabolism. In this study, we used a new methodology for metabolic connectivity with dynamic FDG-PET, and fMRI to evaluate disruptions of resting-state networks using anesthesia.Methods
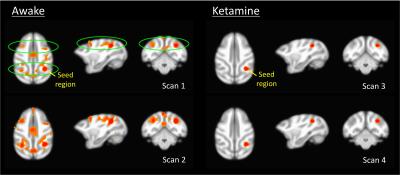
Three Rhesus monkeys (Macatta mulatta) were trained to enter the scanner and remain calm during imaging. All procedures were approved by the local IACUC. Four runs of PET-fMRI data (13.5 min each) were obtained as shown in Figure 2. The 1st and 2nd runs were performed under awake conditions, and the 3rd and 4th runs immediately after injection of 4 mg/kg and 2 mg/kg of ketamine (IV), respectively. All imaging was performed on a 3T Signa PET/MR scanner (GE Healthcare, Waukesha, WI) using a 3-inch surface loop coil. fMRI data acquisition parameters were: 1.8s TR, 20.5 ms TE, 2.67x2.67x3mm pixel size, 70-degree flip angle. With timing matching the fMRI data acquisition, 18F-FDG was infused at a rate of 0.01 mL/s using an MR compatible injector (Medrad Spectra Solaris EP). The total dose of FDG corresponded to ~3.5 mCi. Data analysis for fMRI utilized a conventional seed-based approach using field-map correction, motion correction, bandpass filtering, and smoothing. PET images were reconstructed from list mode data using the following parameters: 30 second frame duration, time-of-flight (VPFX-S), 28 subsets, 2 iterations, 256x256 matrix, 30cm reconstruction diameter. The dynamic PET data were processed in a manner similar to the fMRI data, including motion correction, global brain signal normalization to sensitize the acquisition to local fluctuations, and smoothing. Seed-based resting connectivity correlation was performed in both fMRI and PET acquisitions to identify networks. Effective connectivity between regions was assessed using time-domain conditional Granger causality (CGC)3-4 based on a state-space model5. The magnitude of each conditional pair-wise CGC between network nodes was tested for significance using nonparametric permutation tests corrected for multiple hypotheses.Results
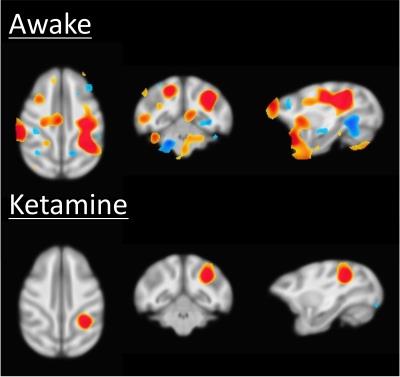
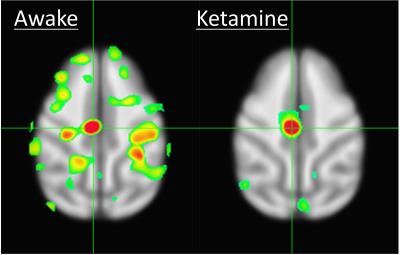
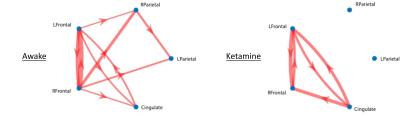
Results of the resting fMRI analysis identifying a fronto-parietal (FP) network are shown in Figure 2. The same network, but with the dynamic PET data is shown in Figure 3. A sensorimotor motor network from dynamic PET is shown in Figure 4. In both the fMRI and PET measures of resting connectivity, administration of ketamine induces a profound reduction in connectivity. Effective connections between brain regions (nodes) for the FP network having significant directional flow are shown in Figure 5. These preliminary results suggest that not only are functional and metabolic connectivity altered, but effective connectivity is also changed by the administration of ketamine.Discussion and Conclusion
To our knowledge, this is the first study that directly compares resting-state networks in the awake vs. ketamine-anesthetized condition. Resting-state connectivity (identified via conventional fMRI and novel PET methods) is profoundly disrupted by the administration of ketamine. This is in contrast to studies that have shown resilient activity in some networks following the administration of different doses of other anesthetics6. Furthermore, we demonstrate methods analogous to resting-state functional connectivity fMRI applied to dynamic measures of PET resting-state metabolic connectivity. These results indicate that in spite of the disrupted hemodynamics known to result from the use of ketamine, which could potentially confound fMRI7, the metabolic connectivity measures obtained via PET, which have been shown not be sensitive to changes in blood flow8, are similarly disrupted. These findings demonstrate a vulnerability of functional, effective, and metabolic connectivity to anesthesia. Further study is necessary to elucidate the effect of anesthesia type, level, and timing, for which an awake model is essential to measure connectivity before, during, and after administration.Acknowledgements
We acknowledge research support from the UW2020: WARF Discovery Initiative, UW SMPH, WNPRC, UW Dept. of Neuroscience, and Dept. of RadiologyReferences
1. Biswal B, Yetkin FZ, Hyde JS, Haughton VM. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995 Oct;34(4):537–41. PMID: 8524021
2. Rogers BP, Morgan VL, Newton AT, Gore JC. Assessing functional connectivity in the human brain by fMRI. Magn Reson Imaging. 2007;25(10):1347–57. PMID: 17499467
3. Roebroeck A, Formisano E, Goebel R. The identification of interacting networks in the brain using fMRI: Model selection, causality and deconvolution. NeuroImage. 2011. p. 296–302. PMID: 19786106
4. Barnett L, Seth AK. Behaviour of Granger causality under filtering: theoretical invariance and practical application. J Neurosci Methods. 2011 Oct 15;201(2):404–19. PMID: 21864571
5. Barnett L, Seth AK. Granger causality for state-space models. Phys Rev E. 2015;91(4):40101.
6. Vincent JL, Patel GH, Fox MD, Snyder a Z, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–6. PMID: 17476267
7. Honey RAE, Honey GD, O’Loughlin C, Sharar SR, Kumaran D, Bullmore ET, Menon DK, Donovan T, Lupson VC, Bisbrown-Chippendale R, Fletcher PC. Acute ketamine administration alters the brain responses to executive demands in a verbal working memory task: an FMRI study. Neuropsychopharmacology. 2004 Jun;29(6):1203–14. PMID: 15100698
8. Villien M, Wey H-Y, Mandeville JB, Catana C, Polimeni JR, Sander CY, Zürcher NR, Chonde DB, Fowler JS, Rosen BR, Hooker JM. Dynamic functional imaging of brain glucose utilization using fPET-FDG. Neuroimage. 2014;100:192–9. PMID: 24936683
Figures