1678
Differential effects of anesthetics on mouse brain connectivity and function as probed by resting-state fMRI and [18]FDG-PET1ICube, CNRS, University of Strasbourg, Strasbourg, France, 2INCI, CNRS, University of Strasbourg, Strasbourg, France, 3Department of Radiology, Medical Physics, University Medical Center Freiburg, Freiburg, Germany, 4Faculty of Biology, University of Freiburg, Freiburg, Germany, 5IPHC, CNRS, University of Strasbourg, Strasbourg, France, 6Engineering Science, Computer Science, and Imaging Laboratory, Integrative Multimodal Imaging in Healthcare, CNRS, University of Strasbourg, Strasbourg, France, Strasbourg, France, 7Department of Biophysics and Nuclear Medicine, University Hospital Strasbourg, Strasbourg, France
Synopsis
Mice resting state functional connectivity (FC) studies are highly attractive given the large number of existing murine models of neuropsychiatric disorders. Currently, most of mouse rs-fMRI studies are carried out under anesthesia, to limit motion and animal distress. Yet, anesthetics affect brain FC. Currently, no reliable awake mouse brain FC reference is available. We compared mouse brain rs-fMRI patterns under medetomidine or isoflurane anesthesia and paralleled the experiments with static 18FDG-PET exams, where the tracer biodistributions occurred under isoflurane, medetomidine anesthesia or in conscious state, reflecting the brain glucose metabolism, as indirect measures of neuronal activity.
Introduction
Resting state fMRI allows investigating the brain networks, capturing features of large-scale neuronal communication. Probing brain FC in mice is crucial for translational research, given the abundancy of mouse models of brain disorders. Anesthesia is required in mouse rs-fMRI to avoid motion and stress although anesthetics influence the neural activity and vascular responses in the brain. Medetomidine and isoflurane are the most commonly used sedatives for rs-fMRI in rodents. Medetomidine is a vasoconstrictor agent and binds to ɑ2-adrenoreceptors, it influences regions with high receptor density1 and affects the connectivity of these regions in a time-dependent manner1,2. Isoflurane has vasodilatory effects5. The lack of a reference conscious brain FC map prevents an exact interpretation of anesthesia impact on the mouse brain. Awake rs-fMRI necessitates time-consuming habituation procedures and it’s not possible to completely rule out the effects of stress3. [18F] Fluorodeoxyglucose positron emission tomography (18FDG-PET) evaluates glucose metabolism; cerebral glucose uptake is closely coupled to synaptic activity4. We used an experimental setup where the biodistribution of 18FDG takes place either under awake condition or under medetomidine or isoflurane, after which the mice are scanned with static-PET. We compared the results from PET imaging with the FC information obtained from rs-fMRI under isoflurane and medetomidine anesthesia for the same cohort of mice. We aimed to fill the gap in knowledge relating to anesthesia effects on the brain metabolism and connectivity as shown by different imaging modalities.Methods
C57BL/6 adult male mice were scanned with rs-fMRI and PET in multiple sessions during the course of 5 weeks.
MRI experiments: First rs-fMRI examination was done under medetomidine (bolus: 0.6 mg/kg, subcutaneous infusion: 0.3 mg/kg/hour) and the second was under isoflurane (1-1.5%). Physiological parameters were closely monitored in each imaging session. MR imaging was done with 7T Bruker Biospec scanner with 86 mm transmission coil and room temperature surface coil adapted for mouse head. GE-EPI pulse sequence was used (27 axial slices, FOV=2.12× 2 cm, matrix=147×87, slice thickness= 0.4 mm, TE/TR= 15 ms /2000 ms, 500 volumes, 0.14×0.23×0.4 mm3 resolution, 16 min acquisition time). Motion correction, spatial normalization and alignment as well as smoothing (FWHM= 0.3×0.5×0.8 mm3) were applied with SPM8. Signal bias correction was performed to eliminate bias due to the use of a surface coil. All the images were normalized into Allen brain atlas and regions of interest (ROIs) were extracted and used in a seed-based correlation analysis. Correlation coefficients were computed between the ROI and the averaged time series of the remaining whole brain and were converted to Z values using Fisher’s r-to-z transformation.
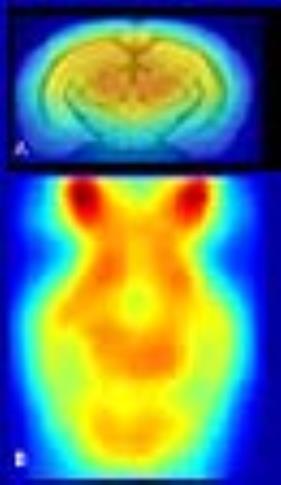
PET experiments: After the first MRI exam, three separate 18FDG-PET exams were performed: first where the biodistribution of the tracer (for 45 min after 8MBq intraperitoneal injections) took place in awake condition, second under isoflurane(1-1.5%) and third under medetomidine (bolus: 0.6 mg/kg; s.c. infusion: 0.3 mg/kg/h); The 15 minute acquisition was done with IRIS (INVISCAN) PET scanner under isoflurane anesthesia for all three. CT images were acquired after PET experiments to help the co-registration on the rs-fMRI data and onto the Allen brain atlas (Fig.1). This allows direct comparison of rsfMRI and PET in the same individuals.
Results and discussion
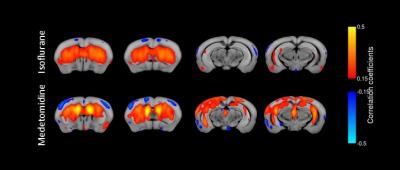
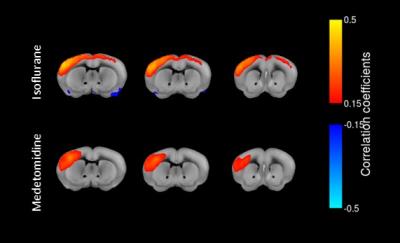
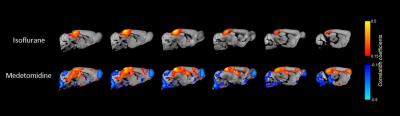
Our rs-fMRI results demonstrate a differential impact of isoflurane and medetomidine on the brain networks. The connectivity patterns are in line with pharmacological properties of these two agents and the distribution of their receptors throughout the brain. Striatal regions have a low density of ɑ2 receptors and indeed, seed analysis showed a higher connectivity with cortical areas in caudoputamen seed region under medetomidine than under isoflurane anesthesia (Fig.2). Additionally, lower interhemispherical cortical connectivity was confirmed for medetomidine using somatosensory cortex seeds as described in previous publications1(Fig.3). Importantly, we observed a strong difference in the patterns of retrosplenial cortex connectivity between two anesthetics, with larger extent of syncronised BOLD signal under medetomidine. RSP is considered the core region in default mode network (DMN) described in both humans and mice. DMN consists of a set of regions that are active in rest and lower their activity during cognitive tasks6 (Fig.4).
In Fig.1, we exemplified the 18FDG traced accumulation in the brain of one animal. Among three experimental conditions, as expected, the highest uptake was observed in the awake condition. Interestingly, isoflurane condition showed higher radiotracer uptake on average than medetomidine although the difference was not statistically significant. We also performed ROI analysis for caudoputamen region which showed uptake ratios similar to that of the whole brain.
Acknowledgements
No acknowledgement found.References
1. Grandjean, J., Schroeter, A., Batata, I. & Rudin, M. Optimization of anesthesia protocol for resting-state fMRI in mice based on differential effects of anesthetics on functional connectivity patterns. NeuroImage 2014; 102: 838–847
2. Nasrallah, F. A., Lew, S. K., Low, A. S.-M. & Chuang, K.-H. Neural correlate of resting-state functional connectivity under α2 adrenergic receptor agonist, medetomidine. NeuroImage 2014; 84: 27–34.
3. Jonckers, E. et al. Different anesthesia regimes modulate the functional connectivity outcome in mice: Anesthesia and Functional Connectivity Outcome in Mice. Magnetic Resonance in Medicine 2014; 72:1103–1112.
4. Shah, D., Deleye, S., Verhoye, M., Staelens, S. & Van der Linden, A. Resting-state functional MRI and [18F]-FDG PET demonstrate differences in neuronal activity between commonly used mouse strains. NeuroImage 2016; 125: 571–577.
5. Fukuda, M., Vazquez, A. L., Zong, X. & Kim, S.-G. Effects of the α 2 -adrenergic receptor agonist dexmedetomidine on neural, vascular and BOLD fMRI responses in the somatosensory cortex. European Journal of Neuroscience 2013; 37:80–95.
6. Raichle, M. E. et al. A default mode of brain function. Proceedings of the National Academy of Sciences 2001; 98:676–682.
Figures