1676
Effect of alfaxalone anesthesia on brain functional connectivity in rhesus monkeys1Yerkes Imaging Center, Yerkes National Primate Research Center, Emory University, Atlanta, GA, United States, 2Division of Neuropharmacology and Neurologic Diseases, Yerkes National Primate Research Center, Emory University
Synopsis
Alfaxalone is suggested to be an optimal anesthetic to examine brain injuries in experimental animals. However, little is known about its impact on neural activity in
anesthetized subjects. In the present study,
adult rhesus monkeys were used to examine its impact on functional connectivity. The results
demonstrate that alfaxalone induces significantly reduced functional
connectivity in the dominant
default-mode network (DMN), inter-hemisphere connectivity in primary
somatosensory cortex and caudate compared to isoflurane. The findings reveal that alfaxalone suppress neural activity more dramatically
than light isoflurane anesthesia in monkeys, suggesting it is ideal for investigating anatomical and microstructural changes in animal models but not good for evaluating neuronal activity with fMRI.
Introduction
Alfaxalone is a synthetic neuroactive steroid anesthetic that can be administered for induction (i.m.) and maintenance (i.v.) of anesthesia. Alfaxalone has been increasingly used in various experimental animals including rodents, dogs and monkeys in recent years due to its fewer side-effects [1]. Prior studies indicated that alfaxalone might not have neuroprotective effect compared to most anesthetics. Therefore it could be a useful anesthetic for assessing the treatment efficacy of neuroprotective drugs using animal models like stroke or traumatic brain injury using MRI as the experimental objectives might not be interfered by anesthesia [2, 3]. However, little is known about its impact on neural activity in the brain of anesthetized animals. Resting state fMRI (rsfMRI) is becoming a robust tool and playing a more important role in preclinical studies of neuroscience and psychiatric disorders [4]. In the present study, the effects of alfaxalone on physiology and functional connectivity (FC) in adult rhesus monkeys were investigated with rsfMRI.Methods
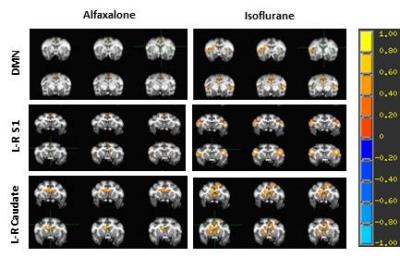
Adult female rhesus monkeys (n=5, 9-13 years old) were employed. Alfaxalone was given as an initial intramuscular injection (5 mg/kg) followed by intravenous infusion (0.125mg/kg/min) to anesthetic effect for about 1 hour. Then Alfaxalone was stopped and followed with isoflurane to keep the animal sedated continuously. Physiological parameters such as O2 saturation, blood pressure, heart rate, respiration rate, body temperature and PaCO2 were monitored continuously and maintained within normal ranges. rsfMRI data were acquired using the multiband EPI sequence [5] (TR/TE=1090 ms/25ms, 550 volumes per scan, spatial resolution= 1.5×1.5×1.5mm3) and started ~15 minutes after animals were moved into the scanner (Siemens 3T Trio with an 8-channel Tx/Rx volume coil). The rsfMRI scan was repeated after the anesthetic was switched to ~0.8% (~0.7 MAC) isoflurane mixed with 100 % oxygen for comparison purpose. Corresponding 3D T1 weighted images and field map images were acquired in the anesthesia transition period. rsfMRI data were preprocessed firstly by field map for image distortion correction with FSL. Slice timing correction, rigid body registration, regressing out signal in white matter and cerebrospinal fluid time series, temporal filtering with 0.009 Hz ~0.0237 Hz band-pass, spatial smooth by a Gaussian blur with 2.5-mm full width at half maximum were performed using a script from AFNI (http://afni.nimh.nih.gov). Anatomical regions of interest (ROI) corresponding to the whole posterior cingulate cortex (PCC), anterior cingulated cortex (ACC), and dorsal/media prefrontal cortex (DMPFC), left/right primary somatosensory cortex (S1) and caudate were selected using AFNI and the monkey brain atlas [4] with T1-weighted images as reference. The averaged time courses of rsfMRI signal in PCC, S1 and caudate were used for seed-based correlation analysis separately. Z transformation was applied to the individual correlation maps to show normalized correlation maps. The averaged z values of connectivity between PCC and ACC or DMPFC, left to right S1 and caudate were examined for statistical differences. All statistical analyses were performed in SPSS 21.0. P-values less than 0.05 were considered statistically significant.Results
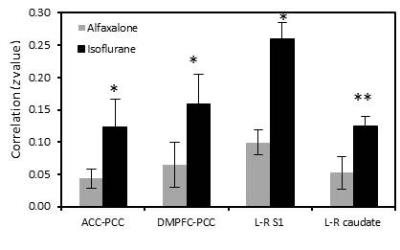
The rsfMRI results showed that the correlation degree of PCC with either DMPFC or ACC, left to right S1 in alfaxalone was less than those z values in isoflurane (Fig. 1, 2), and the difference in PCC-DMPFC, left-right S1 connectivity between two anesthetics was significant (see Fig 1). No significant changes of Mean arterial pressure and heart rates were observed between alfaxalone and isoflurane.Discussion and conclusion
Alfaxalone has been increasingly used in experimental animals including monkeys due to fewer side effects [7, 8]. The general physiological readings were not affected compared to isoflurane. As seen in Fig 1 and 2, the DMN network, cortical-cortical connectivity (S1), subcortical-subcortical connectivity (caudate) pathways were significantly inhibited, suggesting the lower dose (0.125mg/kg/min) alfaxalone has a stronger suppression effect on neural activity compared to the low-dose isoflurane which is usually used in neuroimaging studies to examine the FC or task-based fMRI responses in experimental animals. The anesthetic effects produced by alfaxalone are attributed to the enhancement and modulation of the inhibitory GABA at the GABAA receptor complex [9]. However, the mechanisms of its influence on cerebrovascular dynamics, neural activity and dose-dependence effect are not known and remain to be explored in future studies. In conclusion, alfaxalone shows stronger suppression effect on the brain neuronal activation compared to the low-dose isoflurane. Alfaxalone alone might be an optimal anesthetic for anatomical and microstructural changes in the brain with MRI but not a good anesthetic for fMRI studies in monkeys or other experimental animals. A multimodal anesthetic approach may be a valuable route of exploration for its application in preclinical neuroimaging studies in future projects [10].Acknowledgements
The project was funded by the National Center for Research Resources (P51RR000165) and is currently supported by the Office of Research Infrastructure Programs (OD P51OD011132).References
[1] Wendy Bayldon, J.E.C., Journal of Feline Medicine and Surgery Open Reports (2016); [2] G. Whelan et al. Laboratory animals (1999); [3] Cross AJ et al. Br J Pharmacol (1991); [4] Maria de la Iglesia-Vaya., et al. Novel Frontiers of Advanced Neuroimaging (2013); [5] Preibisch, C., et al., PLoS One (2015); [6] Logothetis, K.S.S.a.N.K., 2007 (First edition). [7] Bakker, J., et al., BMC Vet Res (2013); [8] Casoni, D., Vet Anaesth Analg (2015); [9] Warne, L.N., et al. Vet J (2015); [10] Uhrig, L., et al., Ann Fr Anesth Reanim (2014).Figures