1672
Frequency-dependent hemodynamic response to somatosensory stimulation in ketamine-anesthetized mice1Center for Neuroscience Imaging Research, Institute for Basic Science (IBS), Suwon, Korea, Republic of, 2Health Sciences and Technology, Samsung Advanced Institute for Health Sciences and Technology (SAIHST), Sungkyunkwan Universiy, Suwon, Korea, Republic of, 3Biomedical Engineering, Sungkyunkwan University, Suwon, Korea, Republic of
Synopsis
Optimizing parameters of electrical forepaw stimulation is necessary to investigate biophysical and molecular properties of fMRI signals. However stimulation parameters and anesthesia in mice are not well-defined. Here we introduced ketamine anesthesia in mice. Initially, forepaw stimulation frequency was refined with for CBV-weighted OIS experiments. To translate electrical stimulation parameters from OIS into BOLD fMRI, we tested two stimulation frequencies. Application of our sensory stimulus parameters evoked well-localized and robust BOLD fMRI signals in ketamine anesthetized mice.
Purpose
The demand for fMRI studies in mice have been steadily increasing with advent of transgenic technology. For animal fMRI, anesthesia can be used for reducing animal’s anxiety and minimizing head motions. The choice of anesthesia will modify fMRI responses, and optimal somatosensory stimulation parameters will be changed, which can be dependent on species. Many anesthetics including α-chloralose (GABA receptor agonist) were used in rat fMRI studies 1, but the protocols obtained from rat studies cannot be simply translated into mouse fMRI. Unlike rats, anesthetics and stimulation parameters are not well-defined in mice. Thus, the efficacy of ketamine anesthesia was evaluated in mouse sensory stimulation studies, since ketamine can be used for survival studies 2. First, we examined cerebral blood volume (CBV) weighted responses with optical intrinsic signal imaging (OIS) to a series of electrical forepaw stimulation frequencies. Then, we performed 9.4T BOLD fMRI responding to two forepaw stimulation frequencies.Methods
Experiments were conducted on seven adult male C57BL/6 mice (23-30 g) with the approval of a local Institutional Animal Care and Use Committee for CBV-weighted OIS imaging (n=4) and BOLD fMRI at 9.4T (n=3). Mice were initially anesthetized with a mixture of ketamine and xylazine (100 mg/kg and 10 mg/kg, respectively, Intraperitoneal (IP)). An IP line was then inserted to administer fluids of 5 % dextrose in saline as well as supplementary anesthesia throughout the experiment, which consisted of ketamine alone (30 mg/kg/h), typically commencing about 45 min after induction. During the experiments, animal’s rectal temperature was maintained at 35-37 ℃. A pair of needle (30G) electrodes were placed in left and right forepaw. Electrical pulse stimulation with 0.5 ms width and 0.5 mA current was given to only one paw using a constant current stimulation isolator triggered by a pulse generator.
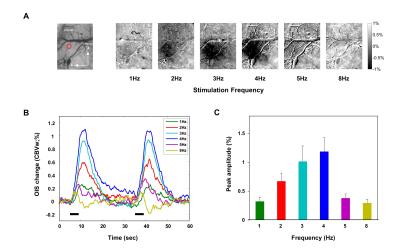
CBV OIS imaging: OIS was measured using MVX-10 microscope with 572 ± 15 nm wavelength filter, which is CBV-weighted. Craniotomy was performed in the contralateral hemisphere to expose the somatosensory cortex 3. Images (FOV = 1.4 mm × 1.1 mm) were captured with CCD camera at a rate of 10 frames/sec. Stimulation parameters stimulation frequencies were 1, 2, 3, 4, 5, and 8 Hz, and stimulation paradigm was 5 s baseline – 4 s stimulation – 26 s baseline – 4 s stimulation – 21 s baseline. Data were analyzed using Matlab.
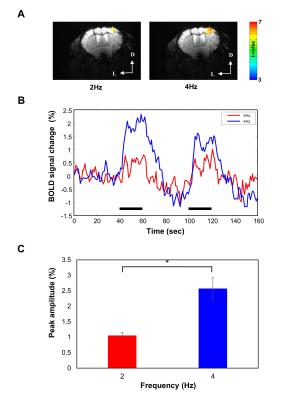
BOLD fMRI: BOLD fMRI was performed using a 9.4T Bruker scanner using GE-EPI (TE = 16 ms, TR = 1000 ms, FOV = 18 mm × 12 mm, imaging matrix = 96 × 64, and 9 contiguous slices with 0.5 mm thickness). Two stimulation frequencies (2 and 4 Hz) were used and stimulation paradigm was 40 s baseline – 20 s stimulus – 40 s baseline – 20 s stimulus – 40 s baseline. AFNI software was used for data analysis.
Results
Frequency-dependent OIS representative subtraction maps were obtained in the contralateral primary somatosensory cortex (Fig.1A). As seen in representative subtraction map, 1, 5, and 8 Hz stimulation did not elicit detectable hemodynamic changes, while 2, 3, and 4 Hz stimulation evoked robust OIS changes. Note that a decrease in 572-nm OIS indicates an increase in CBV. Fig.1B shows time series of CBV-weighted signal changes of the 80 μm-diameter circular ROI (n=4). 2 to 4 Hz stimulation frequencies evoked significant CBV changes beyond > 3 SD of temporal fluctuations from the baseline. The maximum CBV change was observed at 4 Hz stimulation (Fig.1C). Based on these CBV response changes, 2 and 4 Hz stimulation frequencies were chosen for fMRI experiments. Localized BOLD response was detected in the forelimb area of the contralateral primary somatosensory cortex (Fig. 2A). Similar to the results of CBV-weighted OIS experiments, 4 Hz stimulation evoked larger BOLD signal change than that of 2 Hz (Fig.2B and 2C).Discussion and Conclusion
Ketamine is known to be glutamate (NMDA) antagonist, which will reduce neural activity and consequently hemodynamic responses 2. However, in ketamine-anesthetized mice, we observed well-localized, robust CBV-weighted OIS and BOLD fMRI responses in the somatosensory cortex to 4 Hz forepaw stimulation. This frequency tuning is similar to tuning under α-chloralose measured in rats, suggesting that neurovascular coupling may be similar for both anesthetics 1. Our OIS and fMRI studies show that ketamine anesthesia can be used for mouse fMRI studies, which is an important first step for investigating molecular sources of BOLD fMRI with combination of molecular tools. Further studies are needed to optimize stimulation pulse duration and intensity and to investigate reproducibility of longitudinal fMRI studies.Acknowledgements
This work was supported by IBS-R015-D1References
1. Kim T, Masamoto K, Fukuda M, Vazquez AL, Kim SG. Frequency-dependent neural activity, CBF, and BOLD fMRI to somatosensory stimuli in isoflurane-anesthetized rats. Neuroimage. 2010;52(1):224-233
2. Franceschini MA, Radhakrishnan H, Thakur K, Wu W, Ruvinskaya S, Carp S, et al. The effect of different anesthetics on neurovascular coupling. Neuroimage. 2010;51(4):1367-77.
3. Vazquez AL, Fukuda M, Crowley JC, Kim SG. Neural and hemodynamic responses elicited by forelimb- and photo-stimulation in channelrhodopsin-2 mice: insights into the hemodynamic point spread function. Cereb Cortex. 2014;24(11):2908-19.
Figures