1671
Is sevoflurane a viable alternative anaesthetic for functional MRI in mice?1Pharma Research and Early Development, Roche Innovation Center Basel, F. Hoffmann-La Roche Ltd, Basel, Switzerland
Synopsis
In preclinical fMRI, the volatile anaesthetic isoflurane is valued for its ease of use and controllability, and is frequently applied to prevent anxiety and subject motion. Yet, isoflurane increases cerebral blood flow and particularly in mice it limits cerebrovascular response to neural activity, thus curtailing fMRI. Sevoflurane, a close relative to isoflurane, has been reported to be less vasodilatory. Hence, we evaluated whether sevoflurane could serve as an improved anaesthesia modality for fMRI in mice. Perfusion MRI however revealed high basal cerebral perfusion and restricted cerebrovascular reserve capacity in an acetazolamide-induced hypercapnic challenge akin to those previously reported for isoflurane.
PURPOSE
In preclinical MRI studies, anaesthesia is a common procedure applied to prevent anxiety and motion during image acquisition. The volatile anaesthetic isoflurane is among the hypnotics most frequently used in functional MRI studies [1]. It is valued for its ease of administration and maintenance, for providing rapid onset and for its fast and full reversibility. However, isoflurane is a vasodilator and increases cerebral blood flow (CBF) dose-dependently with a particularly strong effect in mice [2]. Consequently, cerebrovascular reserve capacity (CVRC) is curtailed and only a narrow window remains for functional MRI readouts, which are based on neural activity-driven modulations of hemodynamics [3]. Sevoflurane, a close relative of isoflurane, has been shown to exert a significantly lower cerebral vasodilatory effects than isoflurane [4] and maintain global CBF in humans [5]. In pigs, sevoflurane was even reported to reduce CBF [6, 7]. Here, we set out to ascertain whether these propitious properties translate to lab rodents and thus sevoflurane would be a viable alternative anaesthetic for functional MRI in mice.METHODS
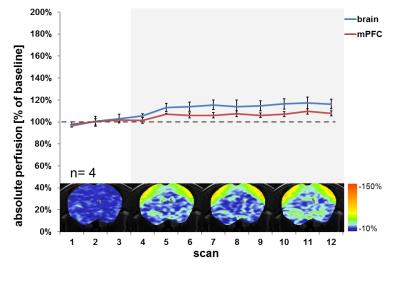
The study was conducted in C57Bl/6 mice (25-35g) with n≥3 individuals per test condition. First, a robust anaesthesia protocol was established for reliable and sustained immobilisation of the animals. Anaesthesia was initially induced in a chamber supplied with 6% of sevoflurane in a gas mixture of 80% air and 20% oxygen, and for maintenance sevoflurane was administered via a face mask to the spontaneously breathing animals. The minimal anaesthetic dose needed was evaluated by reducing the sevoflurane concentration by 1% every 5 min until 4% was reached, followed by a reduction of 0.5% every 15-20 min. Anaesthetic depth was evaluated every 5 minutes by conducting tests for nociceptive (tail pinch and tactile stimulation of the ear canals), withdrawal (toe pinch) or palpebral (medial eye canthus) reflexes and the response to vibrissal (mechanical deflection) and loud sound stimuli (hand clapping) as well as by the breathing rate. In a second step, cerebrovascular reserve capacity was assessed according to a previously established protocol which comprises measurements of absolute brain perfusion at basal conditions and upon i.p. dosing of 30 mg/kg of the carbonic anhydrase inhibitor acetazolamide [3]. Perfusion-MRI was carried out on a BioSpec 9.4T/20cm MR system (Bruker BioSpinMRI, Germany) equipped with a volume resonator for transmission and a surface coil for reception. Perfusion imaging was performed based on continuous arterial spin labelling (CASL) with centred-RARE readout (TR/TE=3s/5.4ms, RARE factor=32, 128x64 matrix, 20mm x 20mm field of view, 0.6mm slice thickness, 8 slices, 2 averages, 3s labelling, 0.4s post labelling delay) [3].Time series of CASL images were obtained by collecting 3 volumes at basal conditions followed by 9 volumes upon acetazolamide injection. For image registration to an anatomical template and brain region-wise quantification of absolute perfusion, T2-weighted anatomical images and T1-maps were also acquired. Group means and standard deviations were calculated.RESULTS AND DISCUSSION
In spontaneously breathing mice, sevoflurane anaesthesia with an induction dose of 5-6% and a maintenance dose of 3% yielded reliable and sustained immobilisation during the ~60 minutes of MRI measurements. This protocol is in line with previous reports on the use of sevoflurane in mice [8, 9]. The maintenance dose of 3% corresponds to approximately ~0.9--fold the minimal alveolar concentration (MAC) and results in a shallow anaesthesia where mild reactions to nociceptive stimuli and withdrawal reflexes were observed. CASL-MRI revealed that basal brain perfusion in mice under sevoflurane anaestesia was at 126 ±4ml/100g/min and thus was in the same range as under isoflurane anaesthesia at the corresponding ~1.1--fold MAC level [3]. The hypercapnic challenge introduced by administration of acetazolamide resulted only in a modest and non-uniform further increase in total and local brain perfusion in these mice (Fig. 1). Hence, we infer that under the present conditions of sevoflurane anaesthesia perfusion is capped and cerebrovascular reserve capacity is nearly exhausted as previously reported for mice under corresponding isoflurane anaesthesia.CONCLUSION
Sustained sevoflurane anaesthesia in spontaneously breathing mice leads to a high basal cerebral blood perfusion, a very small cerebrovascular reserve capacity and thus to an unduly limited hemodynamic response related to neural activity. Hence, sevoflurane does not qualify as an alternative volatile anaesthetic with substantially improved properties for neurovascular coupling-based functional MRI in mice.Acknowledgements
R.K. was supported by the Roche Internship for Scientific Exchange (RiSE) program.References
1 Haensel, J.X., A. Spain, and C. Martin, A systematic review of physiological methods in rodent pharmacological MRI studies. Psychopharmacology (Berl), 2015. 232(3): p. 489-99.
2 Masamoto, K., et al., Dose-dependent effect of isoflurane on neurovascular coupling in rat cerebral cortex. Eur J Neurosci, 2009. 30(2): p. 242-50.
3 Petrinovic, M.M., et al., A novel anesthesia regime enables neurofunctional studies and imaging genetics across mouse strains. Sci Rep, 2016. 6: p. 24523.
4 Matta, B.F., et al., Direct cerebral vasodilatory effects of sevoflurane and isoflurane. Anesthesiology, 1999. 91(3): p. 677-80.
5 Schlünzen, L., et al., Effects of subanaesthetic and anaesthetic doses of sevoflurane on regional cerebral blood flow in healthy volunteers. A positron emission tomographic study. Acta Anaesthesiol Scand, 2004. 48(10): p. 1268-76.
6 Manohar, M. and C.M. Parks, Porcine systemic and regional organ blood flow during 1.0 and 1.5 minimum alveolar concentrations of sevoflurane anesthesia without and with 50% nitrous oxide. J Pharmacol Exp Ther, 1984. 231(3): p. 640-8.
7 Manohar, M., Regional brain blood flow and cerebral cortical O2 consumption during sevoflurane anesthesia in healthy isocapnic swine. J Cardiovasc Pharmacol, 1986. 8(6): p. 1268-75.
8 Puig, N.R., et al., Effects of sevoflurane general anesthesia: immunological studies in mice. International Immunopharmacology, 2002. 2(1): p. 95-104.
9 Cesarovic, N., et al., Isoflurane and sevoflurane provide equally effective anaesthesia in laboratory mice. Lab Anim, 2010. 44(4): p. 329-36.
Figures