1670
Neural and Physiological Responses to Vagus Nerve Stimulation in rats1Biomedical Engineering, Purdue University, West Lafayette, IN, United States, 2Electrical and Computer Engineering, Purdue University, West Lafayette, IN, United States, 3Psychological Sciences, Purdue University, West Lafayette, IN, United States
Synopsis
Vagus nerve stimulation (VNS) is an electroceutical treatment for both brain and visceral organs disorders. To better understand its function in autonomic brain-body interaction, we applied electrical stimulation to the vagus nerve to perturb brain activity and visceral organ physiology. By simultaneously measuring the VNS-induced brain and physiological responses, we separated the afferent neuronal and efferent physiological effects of VNS on the blood oxygen level dependent functional magnetic resonance imaging responses and mapped part of the brain’s autonomic control networks modulated by VNS. This work offers a unique way to characterize the
Purpose
Vagus nerve stimulation (VNS) is an emerging electroceutical treatment for both brain disorders, e.g. depression 1 and epilepsy,2 and chronic diseases in visceral organs such as obesity3 and inflammatory disorders.4 To better understand the autonomic brain-body interaction, we applied localized electrical stimulation to the vagus nerve - a bridging node between the brain and the body, to perturb brain activity and visceral organ physiology. By simultaneously measuring and linking the VNS-induced brain and physiological responses, we aimed to map the brain’s autonomic control networks that modulate physiological conditions. In particular, we aimed to identify and disentangle the afferent neuronal and efferent physiological effects of VNS on the blood oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI) responses, as illustrated in Figure 1.Method
BOLD fMRI was performed on 20 Sprague–Dawley rats (250 - 400 g): two undergoing acute cervical VNS and 18 in an anesthetized resting state. For the former, an MRI-compatible cuff electrode was implanted to the surgically exposed left cervical vagus nerve (Figure 1). During fMRI, the animal was anesthetized with continuous administration of dexmedetomidine (SC-infusion, 0.03 mg/Kg/h) and isoflurane (0.1-0.5% in 1 L/min O2). The fMRI data was acquired in a 7-tesla small-animal MRI system (BioSpec 70/30, Bruker) using a 2-D single-shot gradient-echo echo-planar imaging sequence (1 s repetition time, 15 ms echo time, 55° flip angle, 0.5×0.5×1 mm3 voxel size). For VNS, alternating blocks of VNS were delivered in a 50 s-OFF-10 s-ON cycle (0.1 ms pulse duration, 1 mA pulse current, 10 Hz pulse repetition frequency). Cardiac and respiratory signals were monitored and recorded throughout each experiment.
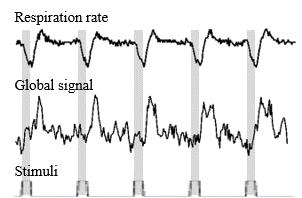
To assess the physiological effects of the VNS, we investigated how the global fMRI signal (averaged from all brain voxels), respiration and cardiac rates were related to the stimulation paradigm. We then regressed out the physiological effects and used a general linear model analysis to assess the residual VNS-induced BOLD activations of likely neuronal origin. Then we compared the VNS-induced activation map with a resting state network that resulted from a seed-based correlation analysis with the seed region placed at one of the VNS-activated regions within the thalamus.
Results
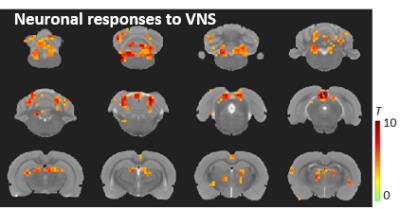
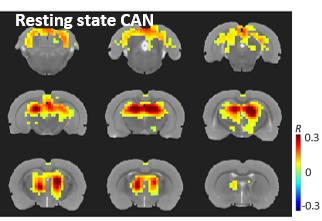
Left cervical VNS decreased the respiration rate and modulated the global fMRI signal; both effects were robust and phase locked to the VNS (Figure 2). In this case, we regarded the global fMRI signal as a surrogate of the non-neuronal and systemic physiological effects of the VNS on the whole-brain BOLD response. Discounting this physiological effect as a nuisance variable in a linear regression model, we detected the neuronal component of the VNS-induced BOLD response. Such neuronal effects were found in the nucleus of solitary tract, dorsal thalamus, and retrosplenial cortex (Figure 3), all of which sub-served a part of the central autonomic control network. This network was also observable in the resting state, as a similar set of regions were found to be temporally correlated to a seed region in the dorsal thalamus (Figure 4).Conclusion
Here, we report a part of intrinsic autonomic control network in the rat brain that is modulated by the left cervical vagus nerve. VNS impacts the BOLD response in the brain in two different ways: 1) It directly triggers specific and neuronal activations at the autonomic control network and 2) it modulates cardiac and respiratory rates and in turn alters the global BOLD signal as a non-neuronal physiological effect. We demonstrate that the neuronal and non-neuronal effects are separable, offering a unique way to image and characterize the brain-activation profile of VNS applied to different locations and with variable parameters.Acknowledgements
This study was funded by National Institutes of Health’s SPARC - Stimulating Peripheral Activity to Relieve Conditions - program (OT2OD023847).References
[1] Rush A J, Marangell L B, Sackeim H A, et al. Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biological psychiatry. 2005: 58(5): 347-354.
[2] Ben-Menachem E. Vagus-nerve stimulation for the treatment of epilepsy. The Lancet Neurology. 2002: 1(8): 477-482.
[3] Berthoud H R. The vagus nerve, food intake and obesity. Regulatory peptides. 2008: 149(1): 15-25.
[4] Borovikova L V, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000: 405(6785): 458-462.
Figures