1669
Defining the challenges in awake rat fMRI1Emory University/Georgia Institute of Technology, Atlanta, GA, United States
Synopsis
Awake rat fMRI has been growing in popularity in recent years because it eliminates confounds associated with the use of anesthesia. However, issues arising from animal restriction have not been completely identified or recognized by the research community. We conducted a pilot study on immobilization training for awake rat fMRI studies and provide some relevant experiences that highlight potential issues to be resolved.
Purpose
Rats have long been utilized for fMRI experiments ranging from basic neurophysiological studies to translational imaging of animal models. Anesthesia is widely used to prevent motion and ideally provide stable physiological conditions. Imaging unanesthetized rats avoids anesthesia effects on neural activity and the vasculature, but introduces challenges due to potential issues arising from rigid restriction [1]. So far only a few groups have reported awake rat fMRI studies [2,3]. What is the major limitation for its broad application in the research community? To answer this critical question, we adopted the restriction strategy suggested in [3], a common paradigm for preparing animals for awake fMRI. We evaluated the frequency of struggling and the condition of the rat during daily restriction training and conducted a preliminary comparison of fMRI resting-state data between the waking state and deep anesthesia.Methods
Eleven Sprague-Dawley rats (~250g, male) were included in this study; 4 were used as controls and 7 underwent acclimation training (restriction/noise) for 7-9 consecutive days with a restraining system (Animal Imaging Research, MA), according to the procedure in [2]. For fMRI studies, the trained animals underwent ~8-min resting state scans during 2% isoflurane (ISO) administration and in the awake state (9.4T Bruker MRI, GE-EPI, TR=500 ms, TE=15 ms, 5 2-mm slices). The data were prepared in SPM (slice timing correction, motion correction and spatial smoothing with 2.5X voxel size FWHM), and bandpass filtered to 0.01-0.15 Hz for functional connectivity analysis. Correlation maps (with or without global signal regression) and power spectra were calculated for seeds in somatosensory cortex and compared across conditions.Results
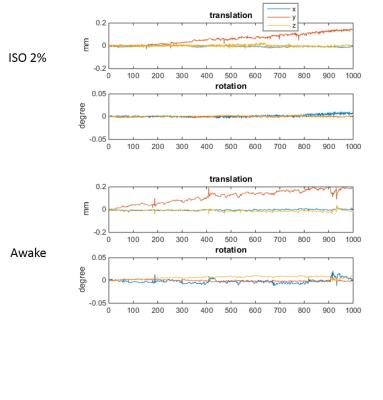
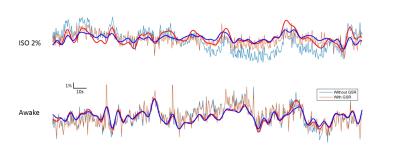
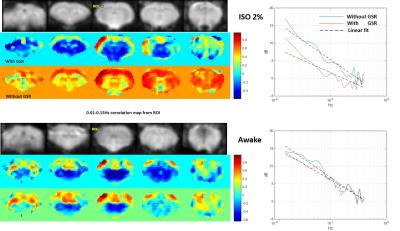
During the daily acclimation, weight gain slowed (2-8g per day) relative to control rats (>12g per day). In 6 of 7 rats, the skin of areas near the ear bars appeared slightly damaged and there were scars at the end of training. Struggling became infrequent after a couple days’ acclimation, with some rats (4 of 7) able to remain still more than consecutive 15min without visible struggling by the end of the training. These rats were considered ready for the resting-state fMRI scan. However, no animal were observed that had no struggling movements during a 30-60 min restriction at the end of acclimation training. One or more 5-10min resting state fMRI awake data sets without struggling movement were obtained from 3 of the 7 trained animals. The data acquired during this stable period had minimal head motion, less than ¼ voxel size (0.4*0.4*2mm3), similar to the data acquired under deep isoflurane (fig. 1). Raw time courses are shown in Fig. 2 and power spectra and functional connectivity maps are shown in Fig. 3.Discussion/ Conclusion
Struggling movements were obvious in the initial days of acclimation, which may increase the risk of pain during restriction, especially near areas where the ear-bars make contact. Ear bars are key for high quality immobilization, but need a proper force to avoid skin damage during struggles. We tried replacing the small-tipped ear bar with an ear pad and found no skin damage but the head position could be changed after a struggling movement. Rats exhibited a reduction in struggling frequency following acclimation. The comparison of data from anesthetized and awake animals shows clear differences in functional connectivity and the effect of global signal regression. Interestingly, global signal regression increased the resemblance between the data acquired under isoflurane and the data acquired in awake animals, similar to previous findings that global signal regression minimized the effects of varying doses of anesthesia [3]. Studies in humans have also found that the global signal is tied to alertness and that caffeine can have similar effects to GSR [4]. Anesthesia is convenient and provides a stable state for animal experiments, but alters brain activity. Awake rat fMRI avoids the confounding effects of anesthesia on neural activity but is time-consuming, possibly stressful and/or painful, and has highly variable results across rats. Until a pain-free restriction method that introduces minimal stress is developed, the assumption that awake rat fMRI represents a “normal” brain state is not well supported. Further efforts are still needed to improve the methods of restriction and acclimation. In the meanwhile, global signal regression should be further investigated as a tool to make data acquired in anesthetized animals more similar to data acquired from awake rats.Acknowledgements
NIH, R01 NS078095References
1. Reed, M.D., A.S. Pira, and M. Febo, Behavioral effects of acclimatization to restraint protocol used for awake animal imaging. Journal of Neuroscience Methods, 2013. 217(1-2): p. 63-66.
2. Febo, M., Technical and conceptual considerations for performing and interpreting functional MRI studies in awake rats. Front Psychiatry, 2011. 2: p. 43.
3. Liang, Z., J. King, and N. Zhang, Anticorrelated resting-state functional connectivity in awake rat brain. Neuroimage, 2012. 59(2): p. 1190-9.
4. King, J.A., et al., Procedure for minimizing stress for fMRI studies in conscious rats. J Neurosci Methods, 2005. 148(2): p. 154-60.
5. Liu, X., et al., The Change of Functional Connectivity Specificity in Rats Under Various Anesthesia Levels and its Neural Origin. Brain Topogr, 2012.
6. Wong, C.W., et al., Anti-correlated networks, global signal regression, and the effects of caffeine in resting-state functional MRI. Neuroimage, 2012. 63(1): p. 356-64.
Figures