1663
Measurements of the flow component of the hemodynamic response function in human cerebral cortex1Neuroscience, Baylor College of Medicine, Houston, TX, United States
Synopsis
Brief cortical neural activity creates changes in local blood flow and oxygen uptake. Functional magnetic resonance imaging can measure this neurovascular coupling as a blood oxygen level dependent (BOLD) signal. The BOLD response to brief stimulation is termed the hemodynamic response function (HRF). We developed a computational model for the BOLD HRF, which predicts that the flow component of the HRF is a simple underdamped sinusoid. To test this prediction, we used arterial spin labeling to measure both CBF and BOLD responses in human cortex with high spatial and temporal resolution. Results confirm a significant flow undershoot in five subjects.
Purpose
In the brain, neural activity evoked by brief stimulation creates changes in local blood flow (CBF) and oxygen uptake. Functional magnetic resonance imaging (fMRI) can measure this neurovascular coupling as a blood oxygen level dependent (BOLD) signal. The BOLD response to brief stimulation is often termed the hemodynamic response function (HRF). We have developed a computational model that accurately predicts both tissue oxygen changes1, and the BOLD HRF2. The model assumes that the CBF response of the HRF corresponds to a simple underdamped sinusoid when measured in gray-matter parenchyma. To test this assumption, we used arterial spin labeling (ASL) to simultaneously measure both CBF and BOLD responses in human cortical tissue with both high spatial and temporal sampling. We also sought to optimize methods to measure the flow component of the HRF.Methods
We measured the BOLD HRF evoked by brief stimulation in 5 subjects. Stimulus was a 2-s presentation of 4-Hz flickering dots colored dots accompanied by bandpass filtered white noise, followed by a 28-s inter-stimulus interval to let the HRF evolve and subside. During the stimulus period, the colored dots were presented sequentially at three different locations upon the screen: left, center, or right. The dot presentations at each location corresponded to a particular color and sound tone: red and low pitch on the left, yellow and medium pitch in the center, green and high pitch on the right. Sequences varied randomly from trial-to-trial (Fig. 1), and subjects were requested to push one of three buttons corresponding the presentation’s position, color, and pitch, while following the stimuli with their eyes. This fairly simple but fast task was moderately challenging. fMRI data was obtained while subjects performed this task using a 3T Siemens Trio scanner equipped with a 32-channel head coil. Acquisition used an ASL sequence, with either a PICORE pulsed3 or pseudo-continuous4 tagging scheme, on 14—18 slices prescribed on a quasi-axial 192-mm FOV oriented roughly parallel to the AC-PC line. TR was 2.5, so that alternating flow and BOLD measurements were obtained every 5 s. We used a dithering method that varied the timing of the stimulus in four steps to improve temporal sampling to 1.25 s. Each session produced 80—96 HRF measurements. MP-RAGE image volumes were also obtained for each subjects, and these volumes were segmented using FreeSurfer to delineate the gray matter. HRF results were then averaged together only within the gray matter. We compared results produced using 2-mm vs. 3-mm voxels, pulsed vs. pseudo-continuous tagging, and with vs. without the use of flow-spoiling gradient lobes designed to suppress signals in larger vessels (flow rates exceeding 6 mm/s).Results
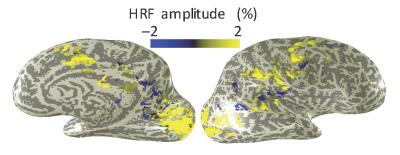
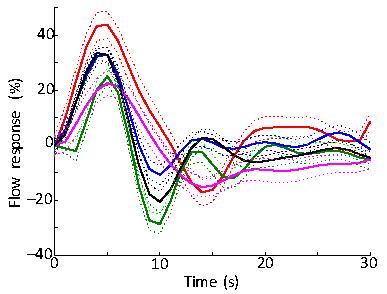
Subjects performed the task well, with mean accuracy of 82%. Strong HRFs were evoked broadly across many brain regions, including portions of visual and auditory cortices (Fig. 2). Best results were obtained using the combination of pseudo-continuous tagging, small voxels, and flow-spoiling gradients. When averaged over primary visual cortex, such data yielded flow HRFs that showed reliable positive lobes followed by a reliable undershoot in all five subjects (Fig. 3). The time-to-peak of the flow response had a mean value of 4.6±0.7 s across subjects, as compared to a mean time-to-peak of 6.1±0.9 s for the BOLD HRF. Pulsed ASL showed a similar flow response, but with lower reliability. Removing the flow suppression, or going to larger voxels both reduced the reliability of the observed dynamics.Discussion
The flow response evoked by brief brain stimulation has an undershoot, which is consistent with our underdamped linear-network model for the flow HRF. Also the flow response peaks earlier than the BOLD HRF, consistent with propagation delays for the oxygenated blood to reach the parenchymal venules, the compartment that dominates the ROLD response. Together, these results confirm that the inertia of blood flow in the larger vessels does play a substantial role in the dynamics of the HRF.Conclusions
The flow component of the BOLD HRF can be accurately resolved by using dithered stimulation combined with pseudo-continuous ASL, high-spatial resolution, gray-matter segmentation, and large-vessel flow suppression. The observed flow HRF shows a reliable undershoot. The ability to simultaneously measure both flow and BOLD opens up the possibility to infer oxygen metabolism in combination with our model for the BOLD response2.Acknowledgements
Work supported by NIH NS R01 NS095933. HL R21 26167539, and NSF BCS 1063774.References
1. J. H. Kim et al., Model of the transient neurovascular response based on prompt arterial dilation, J Cereb Blood Flow Metab 33, 1429—33 (2013); Ress D et al, Front Neuroenerg 1, 1-13 (2009).
2. J. H. Kim, D. Ress, Arterial impulse model for the BOLD response to brief neural activation. Neuroimage 124, 394-408 (2016).
3. E. C. Wong et al., Implementation of Quantitative Perfusion Imaging Techniques for Functional Brain Mapping using Pulsed Arterial Spin Labeling NMR Biomed 10, 237-49 (1997).
4. D. J. J. Wang et al, The Value of Arterial Spin-Labeled Perfusion Imaging in Acute Ischemic Stroke Stroke 43, 1018-24 (2012).
Figures