1661
Correcting for imperfect spin echo refocusing in gas-free fMRI calibration1Montreal Neurological Institute, McGill University, Montreal, QC, Canada, 2Radiology and Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada, 3FMRIB Centre, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 4Cornell MRI Facility, Cornell University, Ithaca, NY, United States
Synopsis
Gas-free calibration is an appealing new alternative for calibrated fMRI. Using simulations, we determined that estimates of the calibration parameter, M, obtained directly by estimating R2’ with asymmetric spin echo imaging, are negatively biased. This is due to imperfect spin echo refocusing of spins diffusing in the extravascular space. When we modelled the spin echo attenuation as a quadratic-exponential decay, the imperfect refocusing effects were accurately accounted for over intermediate to large vessel sizes. When tested in vivo, increases in M were observed when using the quadratic model, however, additional sources of decay also contributed to M.
PURPOSE
Calibration of the BOLD fMRI signal is typically performed using a gas challenge to estimate the calibration parameter, M.1,2 Despite calibrated fMRI’s value, it has not gained widespread use due to the requirement for specialized gas delivery equipment and other factors.3 Gas-free calibration has recently been performed using asymmetric spin echo (ASE)-based measurement of R2' but it was found to underestimate the M-values obtained using hypercapnia.4 This underestimation is postulated to arise from incomplete spin echo refocusing of spins diffusing in the extravascular space. The purpose of this study was to determine how incomplete refocusing of the ASE signal affects the estimation of R2' and how it can be accounted for in order to obtain an accurate estimate of M.METHODS - SIMULATIONS
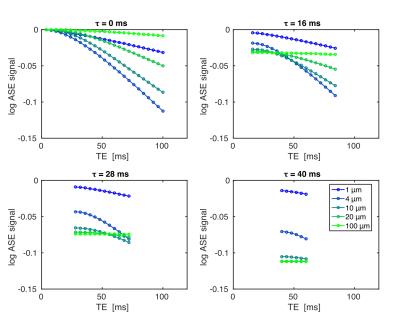
To determine how SE attenuation depends on echo time (TE) and the ASE offset (τ), deterministic simulations of the SE and ASE signals at 3T were run as a function of vessel radius (venous CBV=2%, Hct=40%, SO2=62%). Fig. 1 shows examples of these simulations. With respect to TE, the curves are all relatively well described by a quadratic-exponential decay early on and by linear-exponential decay later, with the time to transition being proportional to vessel radius.
Ignoring the transition to linear-exponential decay, we propose that the SE attenuation can be described by adding a quadratic-exponential decay with rate constant (R2,diff)2 to the conventional ASE signal description:$$S_{ASE}=S_0e^{-R_2\cdot\text{TE}}e^{-R_2^\prime\cdot\tau}e^{-(R_{2,diff})^2(\text{TE}-\tau)^2}.\quad(1)$$Taking the logarithm of the ratio SSE/SASE gives$$ln\left(\frac{S_{SE}}{S_{ASE}}\right)=ln\left(\frac{S_{ASE}(\tau=0)}{S_{ASE}(\tau)}\right)=R_2^\prime\tau+(R_{2,diff})^2\tau^2-2(R_{2,diff})^2\tau\text{TE}.\quad(2)$$From the linear TE-dependence, it should be possible to estimate (R2,diff)2 and R2' if this ratio is measured at two or more TEs. M can then be estimated using$$M=e^{R_2^\prime\cdot\text{TE}}-1\simeq R_2^\prime\cdot\text{TE},\quad(3)$$where TE is the echo time of the functional experiment.
METHODS - IMAGING
Pairs of SE and ASE EPI images were acquired at four TEs from 42–70ms in seven healthy subjects at 3T (31 slices, slice thickness=3.5mm, τ=0/30ms, TR=3600ms, FOV=22.4cm, reps=10). Each set of images was acquired at in-plane resolutions of 2.3mm (N=5) and/or 3.5mm (N=5). Field maps were collected to correct for macroscopic through-plane field inhomogeneity-induced decay.
M was estimated with a hypercapnia run of 5% CO2 (2 min off/on/off). The subjects were imaged with a dual-echo pseudo-continuous ASL sequence to measure the BOLD and cerebral blood flow changes (18 slices, slice thickness=5mm+1mm gap, TR=3600ms, TE1/TE2=9.5/30ms, label duration=1600ms, PLD=900ms, FOV=22.4cm, 64x64 matrix). M-estimates were made using a model-based approach with α/β=0.2/1.3.5
Comparisons of the M-values from the two techniques were done in each subject’s ASL space using four grey matter (GM) regions of interest (ROIs). M was estimated from the ASE images by averaging the signals within each ROI then calculating M from either ln(SSE/SASE) for the early TE or by using multiple TEs with Eqs. 2 and 3.
RESULTS
Fig. 2 shows simulation results comparing the ideal M (Mideal), calculated as the maximum possible gradient echo percent BOLD signal at TE=30 ms, to the estimate using ln(SSE/SASE) at a single TE of 40ms (MASE), as was done previously,4 to the estimate using the quadratic ASE model (q-ASE) with τ=30ms and TEs of 40ms and 50ms (Mq-ASE). MASE underestimated Mideal for all vessel radii, whereas Mq-ASE underestimated Mideal for small to intermediate radii but was in near perfect agreement with it for radii > ~10 μm.
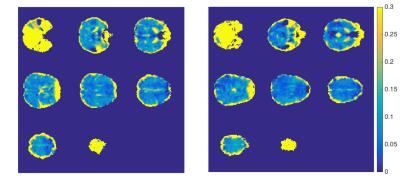
Example M maps from two subjects are shown in Fig. 3. There are hyperintense M-values at the cortical-CSF boundary that likely arise from large blood vessels and CSF.6 Because of this, the brain mask used to produce the GM ROIs was eroded to exclude these voxels from analyses where noted.
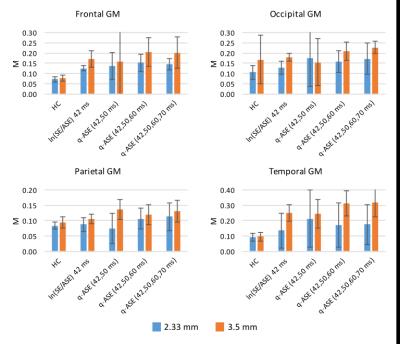
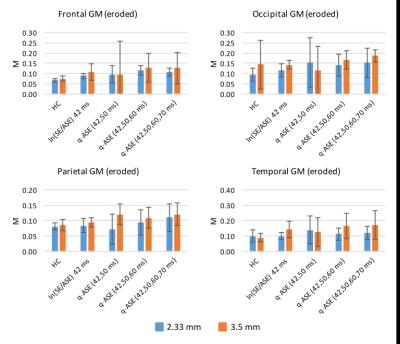
The mean M-values across the ROIs are shown in Figs. 4 and 5 for the original GM ROIs and from the eroded brain mask ROIs, respectively. As expected from the simulations, the in vivo ASE estimates of M were generally found to increase when using the q-ASE model; however, they tended to overestimate the values relative to hypercapnia, in contrast with previous findings.4 Increasing the in-plane resolution from 3.5mm to 2.33mm generally decreased the M values, as did using the eroded brain masks. Together, these findings suggest that both macroscopic field gradients and partial voluming with CSF/large blood vessels were important confounding sources of R2' decay.
CONCLUSION
We have shown that imperfect spin echo refocusing results in an underestimation of the M-value and by characterizing this attenuation as a quadratic decay, the underestimation can be eliminated over a wide range of vessel diameters. When tested in vivo, the q-ASE model did increase the estimates of M in grey matter, making this a promising technique to account for imperfect spin echo refocusing in gas-free fMRI calibration.Acknowledgements
The authors would like to recognize financial support from the Canadian Institutes of Health Research (FDN 143290) and the Campus Alberta Innovates Program.References
[1] T. L. Davis, K. K. Kwong, R. M. Weisskoff, et al. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. PNAS 1998;95(4):1834-9.
[2] P. A. Chiarelli, D. P. Bulte, R. Wise, et al. A calibration method for quantitative BOLD fMRI based on hyperoxia. Neuroimage 2007;37(3):808-20.
[3] N. P. Blockley, V. E. Griffeth, and R. B. Buxton. A general analysis of calibrated BOLD methodology for measuring CMRO2 responses: comparison of a new approach with existing methods. Neuroimage 2012;60(1):279-89.
[4] N. P. Blockley, V. E. Griffeth, A. B. Simon, et al. Calibrating the BOLD response without administering gases: comparison of hypercapnia calibration with calibration using an asymmetric spin echo. Neuroimage 2015;104:423-9.
[5] D. P. Bulte, M. Kelly, M. Germuska, et al. Quantitative measurement of cerebral physiology using respiratory-calibrated MRI. Neuroimage 2012;60(1):582-91.
[6] A. B. Simon, D. J. Dubowitz, N. P. Blockley, et al. A novel Bayesian approach to accounting for uncertainty in fMRI-derived estimates of cerebral oxygen metabolism fluctuations. Neuroimage 2016;129:198-213.
Figures