1654
Characterization of structural and functional connectivity in epilepsy by integrating diffusion and functional tensor imaging1Department of Computer Science, Chengdu University of Information Technology, Chengdu, People's Republic of China, 2Institute of Imaging Science, Vanderbilt University, Nashville, TN, United States, 3Department of Radiology and Radiological Sciences, Vanderbilt University, Nashville, TN, United States, 4Department of Electrical Engineering and Computer Science, Vanderbilt University, Nashville, TN, United States, 5Department of Biomedical Engineering, Vanderbilt University, Nashville, TN, United States, 6Department of Neurology, Vanderbilt University, Nashville, TN, United States
Synopsis
Although previous studies have tried to combine diffusion tensor imaging (DTI) and resting-state functional magnetic resonance imaging (rs-fMRI) in clinic studies, they only use rs-fMRI for gray matter and DTI for white matter pathways. In this study, DTI and rs-fMRI were leveraged for conjoint analysis of structural and functional connectivity in white matter. The results showed that in temporal lobe epilepsy patients, there was an increased functional fractional anisotropy in contralateral fiber bundles, which were accompanied by increases in mean diffusivity in ipsilateral bundles. This reflects complex interactions between disease evolution and compensatory adaption.
Purpose
Typically, diffusion tensor imaging (DTI) and tractography are used to examine tract-specific pathology in white matter (WM) and resting-state functional magnetic resonance imaging (rs-fMRI) is used to study functional connectivity between cortical regions. Intuitively, combining DTI and rs-fMRI may allow for characterization of structure-function relations in neural networks of the human brain. However, their inherent differences in signal biophysical origins and applicable brain regions suggest no direct integration of these two techniques. Based on the concept of functional tensor imaging (FTI) we proposed earlier 1, we establish in this work a new framework for characterizing structural and functional connectivity of WM by combining DTI and FTI. The framework is applied to clinical studies of temporal lobe epilepsy (TLE), which demonstrates, for the first time, asynchronized changes in structural and functional connectivity in the WM of TLE patients.Methods
Subjects: The subjects selected for this study included 24 unilateral TLE patients (8 left and 16 right), and 24 neurologically healthy controls. Localization of the epileptogenic zone was based on standard presurgical evaluation, including structural imaging with MRI, ictal and interictal electroencephalography (EEG), analysis of seizure semiology, and functional imaging with positron emission tomography (PET) 2. Age and gender were matched individually between the two groups, such that each patient had a unique, directly matched control subject..
Image acquisitions: Informed consent was obtained per Vanderbilt University Institutional Review Board guidelines. Whole-brain functional and structural MRI images were acquired on a Philips Achieva 3T MRI scanner (Philips Healthcare, Best, Netherlands). Blood oxygenation level dependent (BOLD) images were acquired with subjects at resting state using a T2*-weighted, gradient echo, echo-planar imaging (EPI) sequence and TR=2 s, TE=35 ms, matrix size=80×80, FOV=240 mm2, 34 slices of 3.5 mm thick with a 0.5 mm gap, and 300 volumes. Diffusion weighted MRI (DW-MRI): A single-shot, spin echo, EPI sequence was used with b=1600 s/mm2, 92 diffusion-sensitizing directions, TR=8.5 s, TE=65 ms, SENSE factor=3, matrix size= 96×96×50, and voxel size=2.5×2.5×2.5 mm3.
Functional image preprocessing: BOLD images were corrected for slice timing and head motion using standard SPM procedures. Physiologic noise was removed by using retrospective image based correction 3. The corrected BOLD images were then normalized spatially to a common MNI (Montreal Neurological Institute) space. Finally, the BOLD images were spatially smoothed using a 3×3×3 mm3 full width, half-maximum Gaussian kernel.
Structural and functional characterization: For each subject, functional tensors were computed voxel-wise from the BOLD images using the method in Ding et al 1. Briefly, by analyzing temporal correlation profiles between the voxel of interest and its neighbors, a functional tensor was constructed which, in resting state signals, captures intrinsic synchronization of BOLD signals. From the functional tensor, functional fractional anisotropy (FFA) was computed as a measure of functional connectivity at a local scale. Meanwhile, a set of 18 major white matter bundles were reconstructed from the DW-MRI data of the same subject using an automated tool TRACULA 4. These bundles were normalized to the MNI space along with diffusion FA (DFA) and mean diffusivity (MD) derived. All structural and functional measures obtained above were averaged along each of these bundles for statistical analysis.
Results and Discussion
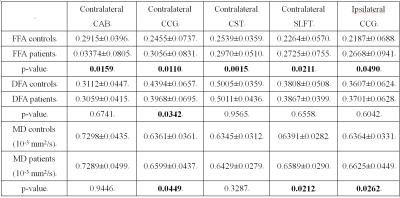
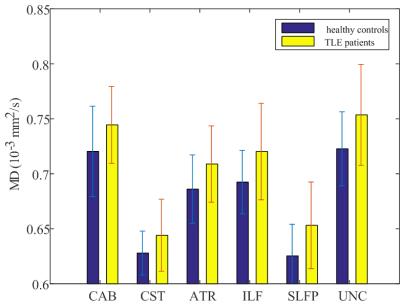
Five fiber bundles in the TLE group, including contralateral cingulum-angular bundle (CAB), cingulum-cingulate gyrus (CCG), corticospinal tract (CST), superior longitudinal fasciculus temporal terminations (SLFT) and ipsilateral CCG, were observed to have an increase in bundle-averaged FFA with p<0.05. Measures of FFA, DFA and MD for these five bundles are statistically summarized in Table 1. As can be seen, the increases in FFA were accompanied by decrease in DFA in the contralateral CCG, and increases in MD in bilateral CCGs and contralateral SLFT with p<0.05. Moreover, there were increases in MD with p<0.05 without significant changes in FFA in several other bundles ipsilateral to disease (see Figure 1), including CAB, CST, anterior thalamic radiation (ATR), inferior longitudinal fasciculus (ILF), superior longitudinal fasciculus parietal endings (SLFP), and uncinate fasciculus (UNC).
Overall, in the TLE group there were FFA increases in contralateral bundles accompanied by MD increases in ipsilateral bundles. These findings agree with previous reports of increased functional connectivity in contralateral cortices and increased structural alterations in ipsilateral fibers in epilepsy 5, 6.
Conclusion
This study represents a very first effort in integrating diffusion and functional tensor imaging for clinical studies of structural and functional connectivity of WM. The findings from this study indicate heterogeneities in functional and structural alterations in TLE, which may be attributable to complicated interactions between pathological evolution and compensatory adaption.Acknowledgements
This work was supported by NIH/NINDS NS035929, NS075270 (Morgan), project J201608 supported by Chengdu University of Information and Technology (CUIT) Foundation for Leaders of Disciplines in Science, National Natural Science Foundation of China under Grant 61602065.References
[1] Ding, Z., Newton, A.T., Xu, R., et al. Spatio-temporal correlation tensors reveal functional structure in human brain. Plos One. 2013; 8(12):e82107.
[2] Morgan, V.L., Rogers, B.P. and Abou-Khalil, B. Segmentation of the thalamus based on BOLD frequencies affected in temporal lobe epilepsy. Epilepsia. 2015; 56:1819-1927.
[3] Glover, G.H., Li, T.Q. and Ress, D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn. Reson. Med., 2000; 44:162-167.
[4] Yendiki, A., Panneck, P., Srinivasan, P., et al. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front Neuroinformatics. 2011; 5(23):12-29.
[5] Bettus, G., Bartolomei, F., Confort-Gouny, S., et al. Role of resting state functional connectivity MRI in presurgical investigation of mesial temporal lobe epilepsy. J. Neurol. Neurosurg. Psychiatr., 2010; 81:1147-1154.
[6] Concha, L., Kim, H., Bernasconi, A., et al. Spatial patterns of water diffusion along white matter tracts in temporal lobe epilepsy. Neurology. 2012; 79:455-462.