1644
Distortion-free fMRI using multi-slice 2D-bSSFP at 7 tesla1CIBM, EPFL, Lausanne, Switzerland
Synopsis
Balanced Stead-State Free Precession (bSSFP) techniques have been proposed as an alternative to GE-EPI to address the problem of co-registration between functional/structural scans at high field. However, 3D imaging is suboptimal for localized fMRI applications, and result in distortions, blurring, and increased sensitivity to motion/physiological noise. Here, we propose to use 2D-bSSFP and cartesian readouts and demonstrate that (i) bSSFP signal characteristics are preserved in a new steady-state, (ii) resting-state fMRI (RS-fMRI) can be performed with 2D-bSSFP, (iii) the 2D-bSSFP temporal SNR levels with that of 3D-bSSFP, resulting in - at least - equal fMRI performances at high field.
PURPOSE
Balanced Stead-State Free Precession (bSSFP) techniques1,2 have been proposed as an alternative to GE-EPI to address the problem of signal drop-outs and co-registration between functional/structural scans at high field3–6. Maintaining the bSSFP steady-state has encouraged single-slice7–9 or 3D imaging using radial10, EPI4, or spiral encoding11. This is suboptimal for localized fMRI applications at high field, and results in either poor coverage for single-slice, or distortions, blurring, and increased sensitivity to motion/physiological noise3 for 3D. Here, we propose to use multi-slice bSSFP (2D-bSSFP) and cartesian readouts and demonstrate that (i) bSSFP signal characteristics are preserved in a new steady-state, (ii) resting-state fMRI (RS-fMRI) can be performed using 2D-bSSFP, (iii) the 2D-bSSFP temporal SNR levels with that of 3D-bSSFP, resulting in –at least- comparable performances for task-fMRI at 7T.METHODS
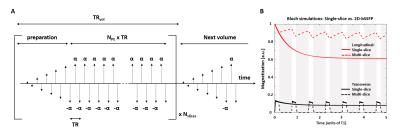
The passband bSSFP acquisition (Fig.1A) was preceded by a “catalyzation” preparation module (10 RF-pulses: linear flip-angle (FA) increase12) before each slice acquisition for rapid convergence towards pseudo-steady-state.
Bloch equations were simulated to investigate the impact of prolonged relaxation between slice repetitions (number of slices:1/2/4/8) on the bSSFP magnetization profile. Simulations covered the offset frequency range [-1/TR–1/TR] and FA=5-40˚. T1 and T2 values were fixed at 2000ms13 and 55ms14, respectively. The impact of realistic tissue modeling, diffusion and RF pulse duration15,16 on spin history was neglected.
Experiments were approved by the local ethics committee and performed on healthy volunteers using a 7T head-only Siemens system and a 32-channel receiver coil (Nova).
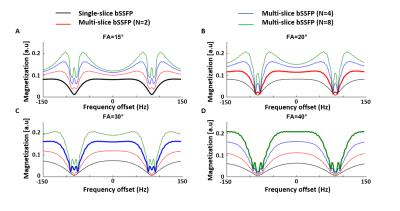
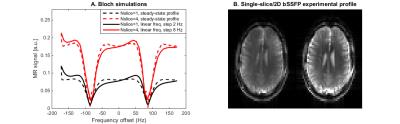
Single- and multi-slice 2D-bSSFP profiles were acquired using: TE/TR=2.9/5.8ms, matrix 52x64, resolution 3.5x3.5mm2, slice thickness 4.5mm, with TRvol=0.375/1.5s and FA=15/30° for one and four slices, respectively. The frequency range [-TR-1:TR-1] was covered using incremental phase cycling steps17. bSSFP profile perturbations due to this phase cycling increase were simulated.
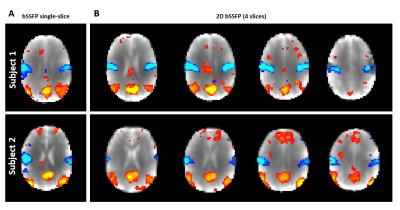
2D-bSSFP RS-fMRI data (3 subjects,10min/run) were acquired using the same parameters. Resting state networks (RSN) were extracted using the melodic toolbox of FSL18 after brain extraction, smoothing (FWHM=6mm) and high-pass filtering.
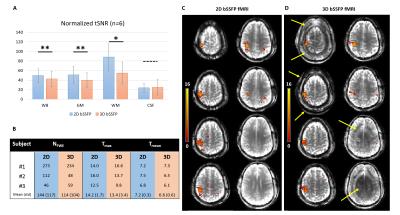
2D- and 3D-bSSFP were compared with matching parameters (5 subjects): TR/TE=5.02/2.51ms, matrix=64x60x8, in-plane/transverse res.=3.5/4.5mm, optimal FA=40/15° for 2D/3D (TRvol=3000/2600ms, fewer preparation pulses for 3D). 2D and 3D normalized tSNR maps (temporal-mean/(temporal-std*TRvol1/2)) were compared at whole-brain level (WB) and in GM, WM and CSF ROIs in mid-brain.
2D- and 3D-bSSFP fMRI performances were evaluated during a finger-tapping task (5 blocks, 30s*[ON/OFF], 3 subjects). After GLM analysis, the number of voxels above 5% FWE threshold (NFWE), and maximum/average T-scores amongst activated voxels (Tmax/Tmean) were compared.
RESULTS
The evolution of the bSSFP transverse/longitudinal magnetization is illustrated in Fig.1B. Compared to conventional bSSFP, higher flip angles were necessary to maximize the 2D-bSSFP passband plateau width (Fig.2A-D), but the magnetization profiles obtained after FA-adjustment were similar. The 2D-bSSFP steady-state benefited from longitudinal magnetization recovery during the acquisition of neighboring slices.
Simulated bSSFP profile perturbations caused by the acquisition scheme were minor (Fig.3A). The experimental 2D-bSSFP profile matched well with conventional bSSFP (Fig.3B, similar transition/passbands). It confirmed the higher 2D-bSSFP signal, although the expected two-fold signal increase was only partially met (~60%). The SNR was sufficient to perform RS-fMRI and extract similar RSN from single-slice and multi-slice-bSSFP data while benefiting from extended coverage (Fig.4).
tSNR was higher with 2D-bSSFP than 3D-bSSFP in GM/WM/WB (Fig.5A, p<0.05). Furthermore, 3D-bSSFP suffered from aliasing along PE2 (Fig.5D) because of imperfect slab selection. Both techniques detected a robust activation cluster in the motor cortex during finger-tapping (Fig.5C-D). Preliminary data (small non-significant differences with n=3) suggest at least equal performances between 2D- and 3D-bSSFP (Fig.5B).
DISCUSSION AND CONCLUSION
Using catalyzation12, we demonstrated that 2D-bSSFP is suitable for resting-state and task-fMRI on a thin slab, and provides distortion-free activation maps at 7T. Costs in temporal resolution due to the need to re-establish steady-state between slices were mitigated by higher signal in the new steady-state. 2D-bSSFP tSNR values were higher than 3D, also as a byproduct of reduced motion/physiological noise, as shown for unaccelerated 2D and 3D-GE-EPI19, and resulting in superior or equal performances for task-fMRI, in addition to reduced image artefacts.
Optimized 3D-bSSFP techniques based on EPI4, spirals11 and/or parallel imaging20 are however expected to surpass 2D-bSSFP SNR-wise, but often at the expense of increased distortion/blurring for rapid read-outs. 2D-bSSFP is better suited for distortion-free localized fMRI, such as retina fMRI8.
Future work will focus on potential whole-brain coverage and higher spatial resolution, combining 2D-bSSFP with in-slice (GRAPPA) and through-slice (SMS) acceleration, as a less motion-sensitive alternative to 3D-bSSFP.
Multi-slice 2D-bSSFP fMRI was demonstrated as a promising tool for distortion-free localized fMRI at ultra-high field.
Acknowledgements
This work was supported by the Centre d'Imagerie Biomédicale (CIBM) of the University of Lausanne (UNIL), the Swiss Federal Institute of Technology Lausanne (EPFL), the University of Geneva (UniGe), the Centre Hospitalier Universitaire Vaudois (CHUV), the Hôpitaux Universitaires de Genève (HUG) and the Leenaards-Jeantet Foundations.References
1. Scheffler K, Lehnhardt S. Principles and applications of balanced SSFP techniques. Eur. Radiol. 2003;13:2409–2418. doi: 10.1007/s00330-003-1957-x.
2. Bieri O, Scheffler K. Fundamentals of balanced steady state free precession MRI. J. Magn. Reson. Imaging 2013;38:2–11. doi: 10.1002/jmri.24163.
3. Miller KL, Smith SM, Jezzard P, Wiggins GC, Wiggins CJ. Signal and noise characteristics of SSFP FMRI: A comparison with GRE at multiple field strengths. Neuroimage 2007;37:1227–1236. doi: 10.1016/j.neuroimage.2007.06.024.
4. Chappell M, Håberg AK, Kristoffersen A. Balanced steady-state free precession with parallel imaging gives distortion-free fMRI with high temporal resolution. Magn. Reson. Imaging 2011;29:1–8. doi: 10.1016/j.mri.2010.07.007.
5. Scheffler K, Ehses P. High-resolution mapping of neuronal activation with balanced SSFP at 9.4 tesla. Magn. Reson. Med. 2016;76:163–171. doi: 10.1002/mrm.25890.
6. Zhong K, Leupold J, Hennig J, Speck O. Systematic investigation of balanced steady-state free precession for functional MRI in the human visual cortex at 3 Tesla. Magn. Reson. Med. 2007;57:67–73. doi: 10.1002/mrm.21103.
7. Cheng JS, Gao PP, Zhou IY, Chan RW, Chan Q, Mak HK, Khong PL, Wu EX. Resting-state fMRI using passband balanced steady-state free precession. PLoS One 2014;9. doi: 10.1371/journal.pone.0091075.
8. Muir ER, Duong TQ. Layer-specific functional and anatomical MRI of the retina with passband balanced SSFP. Magn. Reson. Med. 2011;66:1416–1421. doi: 10.1002/mrm.22935.
9. Park SH, Kim T, Wang P, Kim SG. Sensitivity and specificity of high-resolution balanced steady-state free precession fMRI at high field of 9.4T. Neuroimage 2011;58:168–176. doi: 10.1016/j.neuroimage.2011.06.010.
10. Benkert T, Ehses P, Blaimer M, Jakob PM, Breuer FA. Fast isotropic banding-free bSSFP imaging using 3D dynamically phase-cycled radial bSSFP (3D DYPR-SSFP). Z. Med. Phys. 2016;26:63–74. doi: 10.1016/j.zemedi.2015.05.001.
11. Jin HL, Dumoulin SO, Saritas EU, Glover GH, Wandell BA, Nishimura DG, Pauly JM. Full-brain coverage and high-resolution imaging capabilities of passband b-SSFP fMRI at 3T. Magn. Reson. Med. 2008;59:1099–1110. doi: 10.1002/mrm.21576.
12. Deshpande VS, Chung YC, Zhang Q, Shea SM, Li D. Reduction of transient signal oscillations in true-FISP using a linear flip angle series magnetization preparation. Magn. Reson. Med. 2003;49:151–157. doi: 10.1002/mrm.10337.
13. Wright PJ, Mougin OE, Totman JJ, et al. Water proton T1 measurements in brain tissue at 7, 3, and 1.5 T using IR-EPI, IR-TSE, and MPRAGE: results and optimization. MAGMA 2008;21:121–130. doi: 10.1007/s10334-008-0104-8.
14. Yacoub E, Duong TQ, Van De Moortele P-F, Lindquist M, Adriany G, Kim S-G, Ugurbil K, Hu X. Spin-echo fMRI in humans using high spatial resolutions and high magnetic fields. Magn. Reson. Med. 2003;49:655–64. doi: 10.1002/mrm.10433.
15. Bieri O, Scheffler K. SSFP signal with finite RF pulses. Magn. Reson. Med. 2009;62:1232–1241. doi: 10.1002/mrm.22116.
16. Bieri O, Scheffler K. Effect of diffusion in inhomogeneous magnetic fields on balanced steady-state free precession. NMR Biomed. 2007;20:1–10. doi: 10.1002/nbm.1079.
17. Patterson S, Beyea SD, Bowen C V. FMRI using high flip-angle alternating steady state balanced SSFP supported by Monte Carlo studies. In: Proc. Intl. Soc. Mag. Reson. Med. 19. ; 2011. p. 1630.
18. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. In: NeuroImage. Vol. 23. ; 2004. doi: 10.1016/j.neuroimage.2004.07.051.
19. Reynaud O, Narsude M, Jorge J, Marques JP, Gruetter R, Van Der Zwaag W. Comparison of physiological signals characterization efficiency of SMS-EPI and 3D-EPI-CAIPI at 7T. In: Organization for Human Brain Mapping. Geneva; 2016. p. 2074.
20. Jou T, Patterson S, Pauly JM, Bowen C V. Fat-suppressed alternating-SSFP for whole-brain fMRI using breath-hold and visual stimulus paradigms. Magn. Reson. Med. 2016;75:1978–1988. doi: 10.1002/mrm.25797.
Figures