1634
Simultaneous brain and spinal cord fMRI using slice-based dynamic shimming1Bioengineering, Stanford University, Stanford, CA, United States, 2Stanford University, Stanford, CA, United States
Synopsis
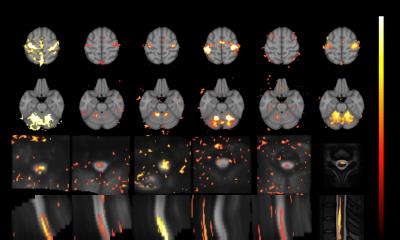
Simultaneous brain and spinal cord fMRI would be beneficial for studying the nervous system, but is challenging due to the poor B0 homogeneity and small size of the spinal cord. Here, we used dynamic shimming and reduced FOV imaging to address these issues. We obtained improved T2*-weighted images of brain and spinal cord with high clarity. We validated the method for fMRI applications using an fMRI experiment with a fist-clenching task, in which we obtained activation in all regions expected to be involved in the task, including the motor cortices, cerebellum, and C6-C8 spinal cord region.
Purpose
Simultaneous fMRI of the brain and spinal cord would allow the study of interactions between these organs in a range of normal and abnormal states, but is challenging to perform for various reasons. B0 field inhomogeneity in the spinal cord region produces image distortion and signal loss with long readouts, which are needed in spinal cord fMRI because high resolution is necessary due to its small size. In addition, spinal cord motion from cardiac and respiratory activity produces unwanted signal fluctuations and motion registration challenges1. Previously, Finsterbusch et al used dynamic shimming, with one set of shims for the brain and one set for the spinal cord to mitigate off-resonance effects, and a reduced FOV in the spinal cord for high-resolution, using saturation pulses to avoid aliasing2. A limitation of their approach is inadequacy of B0 compensation over the spinal cord, where the field varies non-linearly along its length. In addition, the saturation pulses used intersected below the region of spinal cord imaged, prohibiting imaging of lower regions. Here, we use a slice-based dynamic shimming approach3-4 to minimize tradeoffs in field homogeneity and a multi-dimensional RF pulse5 to excite only the local spinal cord region to avoid aliasing with a reduced FOV. We dynamically adjust the readout gradients to obtain different resolutions in the brain and spinal cord, and use parallel imaging for shorter readouts. We validate the method using a fist-clenching task, and obtain functional activation in the anticipated regions of the brain and spinal cord.Methods
For the dynamic shim calculation, two B0 field maps are acquired: an axial map with 18 brain and 12 spinal cord slices and a sagittal map with 1 slice through the brain and spinal cord, acquired during a breath-hold to minimize breathing-related B0 fluctuations6. The calculation minimizes the total off-resonance in the L2 sense in all voxels within a brain and spinal cord mask using one set of static shims (the second-order gradients xy, yz, xz, x2-y2, and z2-(x2+y2)/2), and a separate set of dynamic shims (the linear gradients x, y, z and a frequency offset Δf) for each slice3-4 (Figure 1). The shim field is linear with respect to the shim amplitudes, and thus can be written as the linear system o = Es, where o is the negative of the axial field map, E is a spatial-encoding matrix, and s are the shim amplitudes. To account for through-slice dephasing, for each axial slice, we fit a 2nd order polynomial along z to a local 8 cm region in the sagittal map, with the linear and offset terms at the slice location giving the z and Δf shims for that slice. The static shim field geometries differ from those given by their names, e.g. the xy shim does not have a perfect xy geometry. To account for this, we modeled each static shim field as a linear combination of all the shims, stored the coefficients in a matrix, and used a matrix inversion to obtain the shims needed to be specified [4]. The fMRI sequence parameters were: EPI with GRAPPA acceleration7 (R = 2), flip angle = 80°, FOV for the brain/spinal cord = 22 cm / 8 cm, matrix size = 64 × 64, readout bandwidth = ±125 kHz, TE/TR = 30 ms/2.4 s, # slices = 30 (18 brain, 12 spinal cord in the C5-C8 region), and slice thickness/spacing = 4 mm/2 mm. We used a slice-select excitation pulse in brain slices and an EPI pulse5 in spinal cord slices. To validate the method for fMRI applications, we performed a fist-clenching task with 30 s blocks. We performed motion registration8 using the Spinal Cord Toolbox9-10, physiological denoising with RETROICOR11, and fMRI analysis12-13 (including a general linear model) using FSL14.Results and Discussion
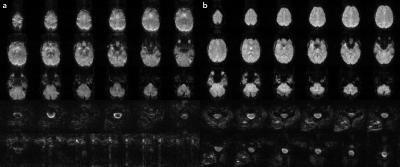
Using slice-based dynamic shimming and reduced FOV imaging with a multi-dimensional RF pulse, we obtained improved B0 homogeneity (Figure 1) and T2*-weighted images in the brain and spinal cord (Figure 2), and showed functional activation in the motor cortex, cerebellum, and C6-C8 spinal cord region from a fist-clenching task (Figure 3). Currently, the implementation acquired axial slices, but individually-oriented slices perpendicular to the spinal cord length are desirable to minimize through-plane field variation and intravoxel effects, and would enable imaging longer sections of the spinal cord. To avoid aliasing of the EPI pulse, the response aliases must lie outside the object, which may be difficult in wider regions of the body, and may require modifying the pulse parameters or using methods such as parallel excitation15 or saturation pulses.Acknowledgements
General Electric Healthcare. NIH Grant: P41 EB0015891.References
1. Stroman PW, et al. The current state-of-the-art of spinal cord imaging: Methods. Neuroimage 2014;84:1070–1081.
2. Kim DH, Adalsteinsson E, Glover GH, Spielman DM. Regularized higher-order in vivo shimming. Magn Reson Med 2002; 48:715-722.
3. Morrell G, Spielman D. Dynamic shimming for multi-slice magnetic resonance imaging. Magn Reson Med 1997; 38.3:477-483.
4. Finsterbusch J, Sprenger C, Büchel C. Combined T2*-weighted measurements of the human brain and cervical spinal cord with a dynamic shim update. NeuroImage 2013;79:153–161.
5. Pauly J, Nishimura D. Macovski A. A k-space analysis of small-tip-angle excitation. J Magn Reson 1989;81:43–56.
6. Griswold MA, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med 2002; 47:1202–1210.
7. Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162-7.
8. Jenkinson M, Beckmann CF, Behrens, TE, Woolrich, MW, Smith SM. FSL. Neuroimage 2012;62:782–790.
9. Jenkinson M, Bannister P, Brady JM, Smith SM. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage 2002;17(2):825-841.
10. Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in fMRI. Neuroimage 2003;20:1052–1063.
11. Woolrich M. Robust group analysis using outlier inference. Neuroimage 2008;41:286–301.
12. Cohen-Adad J, Rossignol S, Hoge RD. Slice-by-slice motion correction in spinal cord fMRI: SliceCorr. Proceedings of the 17th Annual Meeting of the International Society for Magnetic Resonance in Medicine, Honolulu, USA, 2009.
13. Cohen-Adad J, et al. Spinal Cord Toolbox: an open-source framework for processing spinal cord MRI data. 20th Annual Meeting of the Organization for Human Brain Mapping, Hamburg, Germany, 2014.
14. van Gelderen P, de Zwart JA, Starewicz P, Hinks RS, Duyn JH. Real-time shimming to compensate for respiration-induced B0 fluctuations. Magn Reson Med 2007;57.2:362-368.
15. Zhu Y. Parallel excitation with an array of transmit coils. Magn Reson Med 2004;51:775–84.
Figures