1633
High-resolution semi-adiabatic spin-echo fMRI at 7T.1Bioengineering, Stanford University, Stanford, CA, United States, 2Stanford University, Stanford, CA, United States, 3Radiology, Stanford University, Stanford, CA, United States, 4Radiology, Neuroscience, Mount Sinai, NY, United States
Synopsis
Spin-echo fMRI provides greater functional specificity than gradient-echo fMRI, but suffers from lower sensitivity. At higher field strengths, the spin-echo signal contribution from smaller capillaries, closer to the site of neural activity, increases. A major challenge of MRI at high field strengths, however, is increased B1 inhomogeneity, which can impact the performance of RF pulses, in particular refocusing pulses. Here, we use an adiabatic refocusing pulse and a matched-phase excitation pulse to perform high-resolution spin-echo fMRI at 7T. We compare the results to a similar gradient-echo acquisition, and show sharper activation better localized to gray matter in the spin-echo results.
Purpose
Studies have shown that spin-echo (SE) fMRI provides higher functional specificity than gradient-echo (GE) fMRI since the contrast arises mainly from smaller vessels closer to the site of neural activity instead of vessels of all sizes1. However, SE fMRI suffers from lower sensitivity compared to GE fMRI, and is thus often performed at higher field strengths (7T and above). This is to leverage not only the intrinsically higher SNR, but also the increased signal contribution from extravascular spins, which are better indicators of neural activity1. SE fMRI at higher field strengths, however, poses various challenges: 1) increased B1 inhomogeneity, which is particularly problematic for SE sequences, which require accurate refocusing pulses; 2) increased SAR deposition, which limits the number of slices or repetition time1; and 3) the requirement for higher spatial resolution to resolve sharper function activation, which leads to longer readouts, and hence greater susceptibility effects and signal decay. Hyperbolic-secant (HS) adiabatic pulses2 are robust to B1 inhomogeneity above a given threshold, but produce quadratic phase across the slice-profile, resulting in signal loss. This phase cannot be refocused with linear gradients, but an excitation pulse can be designed3 using the SLR algorithm4 such that the phase responses of the two pulses cancel5. Here, we use a matched-phase excitation and adiabatic refocusing pulse, and SENSE parallel imaging6 to perform high-resolution SE fMRI during a breath-hold task and visual + sensory-motor task, and compare the results to similar GE fMRI acquisitions.Methods
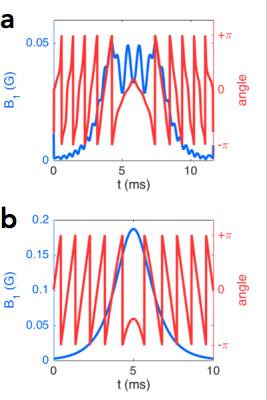
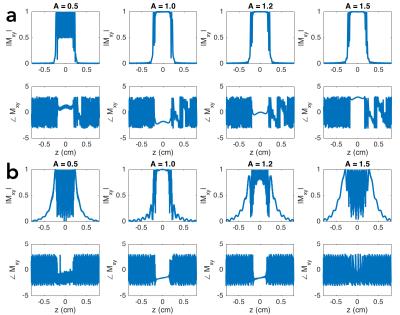
For the SE sequence, the excitation pulse was designed using the SLR algorithm, with parameters: pulse length = 11.7 ms, bandwidth = 2.2 kHz, and quadratic phase = 8.3 rad (defined as the phase difference between the center and edge of the bandpass). The HS pulse was designed with parameters: pulse length = 10 ms, mu = 6.9 and beta = 1 rad/ms. The slice-select gradient amplitudes for the two pulses were identical (1.29 G/cm) so that the same region is selected in the presence of off-resonance (see pulse designs in Figure 1 and response profiles in Figure 2). The acquisition was performed using an SENSE-accelerated6 EPI sequence, with acceleration factor R = 2, FOV = 22 cm, # slices = 5, matrix-size = 110 × 110, and readout BW = ±125 kHz. The GE sequence used a standard slice-select excitation pulse and the same acquisition method. The echo times for the SE and GE sequences were 56 ms and 25 ms, respectively, with repetition time = 2 s. To assess the relative merits of SE and GE fMRI, two experiments were performed with IRB approval: a 10 min breath-hold task with 20 s rest and 10 s breath-hold blocks, and a 5 min 30 s visual (flashing checkerboard) + sensory-motor (fingertapping) task with 30 s ON and OFF blocks. Analysis was performed using GLM, in which the task regressor was the block design convolved with the hemodynamic response function, and the nuisance regressors were polynomials up to order 4.Results and Discussion
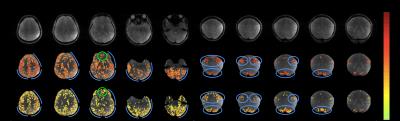
Figure 3 shows the activation maps for the SE and GE experiments. The SE activation is more localized to gray matter (see blue curves in the breath-hold experiment) and sharper (see blue circles in the visual + sensory-motor experiment). These results agree with the theoretical increased specificity of SE fMRI, in which BOLD contrast arises closer to the site of neural activity. In addition, the SE fMRI maps retain activation in regions with large susceptibility gradients (see green circles near the frontal sinuses). The main limitation of this method is high RF power deposition from the adiabatic refocusing pulse, which limits the rate of acquisition, here to about 6 slices every 2 s. A potential solution is to increase the adiabatic pulse length and thus lower the peak B1 amplitude, but this would require a shorter readout, perhaps e.g. using a higher acceleration factor. Another approach is B1-shimming7, i.e. design non-adiabatic RF pulses to compensate for B1 inhomogeneity. The semi-adiabatic SE fMRI sequence we have developed offers an alternative for capturing functional BOLD signal with higher specificity but lower sensitivity than GE fMRI, making it better suited for applications which are not severely SNR-limited, such as sensory stimulation or block-design experiments. The sequence addresses one of the major challenges of high-field imaging, increased B1 inhomogeneity, and will enable researchers to resolve more in-depth and subtle characteristics of brain function.Acknowledgements
General Electric Healthcare. NIH Grant: P41 EB0015891.References
1. Norris DG. Spin-echo fMRI: The poor relation? NeuroImage 2012;62:1109-15.
2. Tannús A, Garwood M. Adiabatic Pulses. NMR in Biomedicine 1997;10: 423–434.
3. Schulte RF, Tsao J, Boesiger P, Pruessmann KP. Equi-ripple design of quadratic phase RF pulses. J Magn Reson 2004;166:111-122.
4. Pauly J, Le Roux P, Nishimura D, Macovski A. Parameter relations for the Shinnar-Le Roux selective excitation pulse design algorithm [NMR imaging]. IEEE T Med Imaging 1991;10.1:53-65.
5. Balchandani P, Khalighi MM, Glover G, Pauly J, Spielman D. Self-refocused adiabatic pulse for spin echo imaging at 7 T. Magn Reson Med 2012;67(4):1077-85.
6. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: Sensitivity encoding for fast MRI. Magn Reson Med 1999;42:952–962.
7. Van den Bergen B, Van den Berg C, Bartels LW, Lagendijk J. 7T body MRI: B1 shimming with simultaneous SAR reduction. Phys Med Biol 2007;52:5429–5441.
Figures