1632
Phase-offset Multiplanar Echo-planar Imaging (POMP-EPI) to Accelerate fMRI Acquisition without Parallel Imaging1Stanford University, Stanford, CA, United States
Synopsis
Simultaneous-multislice (SMS) has significantly improved fMRI acquisition due to its increase in SNR efficiency. However, SMS requires parallel-imaging in the slice-direction, which may be inapplicable due to the scarcity of receiver-coils with adequate sensitivity in the slice-direction. Hence, we propose the POMP-EPI method, which accelerates fMRI acquisition without using parallel-imaging. POMP-EPI works by using Gz-gradient blips in an EPI sequence to shift each of the simultaneously excited slices into different regions of an extended FOV, such that no aliasing occurs, resulting in simple reconstruction of images, shorter TRs, and increased SNR efficiency.
Purpose
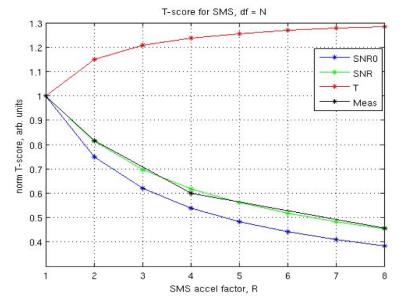
Simultaneous-multislice (SMS) improves the efficiency of fMRI and other imaging techniques that require high SNR (Fig1). However, current SMS techniques require parallel-imaging for spatial-encoding in the slice-direction, exploiting differences in coil sensitivities, but this may not be achievable due to several reasons: (1) there is limited availability of receiver-coils with sufficient sensitivity in the slice-direction, which would amplify g-factor effects and degrade the performance of slice-unaliasing, (2) larger patients may not fit in the multi-channel receiver head coils, and (3) patients with implanted devices can only be imaged with transmit-receive head coils as opposed to receive-only coils, used in parallel-imaging, due to risks of the devices coupling to the body-transmit coils and exceeding “safe” SAR-values. Hence, we propose to accelerate fMRI acquisition without parallel-imaging via the phase-offset multiplanar echo-planar imaging (POMP-EPI) method, which shifts each of the simultaneously excited slices into a different region of an extended FOV, such that there is no aliasing of the slices, resulting in simple reconstruction of images and higher acquisition efficiency.Background and Theory
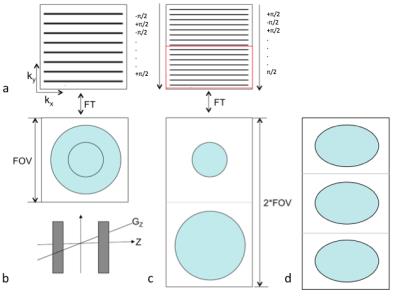
Before parallel-imaging, techniques using phased-multiband RF pulses to simultaneously excite multiple slices, such as POMP1 and Hadamard-encoded excitation2, have shown to increase SNR by a factor of √Nslices, where Nslices is the number of simultaneously-excited slices. However, this increase in SNR comes at a cost of additional RF excitations. Specifically, the idea for POMP is to excite multiple slices such that each slice has a unique phase-gradient in k-space, and is thus shifted to a different region of the (extended) FOV in image space due to the Fourier shift-theorem. Recent developments in parallel-imaging led to the rise of SMS, which uses differences in coil sensitivities in the z-direction to unalias the overlapping slices via methods such as GRAPPA3 or SENSE4. This leads to an SNR efficiency improvement of √(Nslices/g), where g is the geometry-factor. Hence the SNR improvement is highly dependent on the geometries and sensitivities of the coil array. Previous studies such as MS-CAIPI5 and blipped-CAIPI6, have shown that controlled-aliasing of the slices mitigates the g-factor penalty in parallel-imaging.
To increase SNR efficiency without parallel-imaging, our proposed method POMP-EPI uses an EPI-trajectory for its fast imaging speed, while extending the FOV in the phase-encode direction and using gradient-blips in the slice-direction similar to those in blipped-CAIPI (concurrently with phase-encode blips) to impose unique phase-gradients in k-space for each slice. Extending the FOV while maintaining the resolution requires additional phase-encodes, leading to a longer readout, but due to the oblong shape of the brain, the readout only needs to be doubled in order for three slices to fit (Fig2). This allows the TR in fMRI to be shortened in the absence of parallel imaging.
Methods
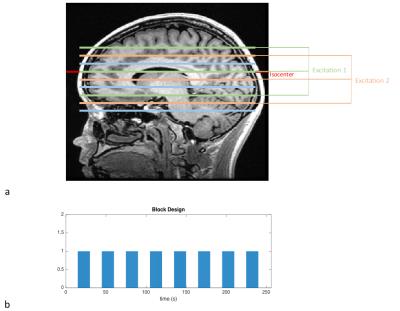
The POMP-EPI sequence was programmed in EPIC and tested on a 3T-GE-Discovery MR-750 scanner. The RF pulse, a modulated-windowed-sinc, excites three slices spaced 40mm apart (Fig3a), so only 10 excitations are required per TR to acquire 30 brain slices. Matrix-size/TE/TR/flip-angle/FOVX/FOVY/slice-thickness/BW = 60x120/45ms/1000ms/62°/22cm/44cm/4mm/125kHz was used. For comparison, a conventional EPI sequence with matrix-size/TE/TR/flip-angle/FOVx/FOVY/slice-thickness/BW = 60x60/30ms/1800ms/75°/22cm/22cm/4mm/125kHz was acquired. Minimum TR and the Ernst-angle were chosen to optimize acquisition efficiency under the same scan time.
Two fMRI experiments using a sensory task were performed to validate our theory. A block-trial paradigm of length 256s (Fig3b) with a contrast-reversing checkerboard visual stimulus was used. Under the same scan time, 256 times frames (256s/1s) were acquired for POMP-EPI, while only 143 time frames (256s/1.8s) fit in the EPI scan.
Phase-correction was applied using the reference scans (acquired prior to the fMRI scans) to remove Nyquist-ghosts. Additional phase (linear along phase-encoding direction) was applied to raw POMP-EPI data to correct for slices not at isocenter. GLM analysis was performed in MATLAB to generate t-score maps for fMRI activation (Fig4), in which the task regressor was the block design convolved with the hemodynamic response function and the nuisance regressors were polynomials up to order 2.
Results and Discussion
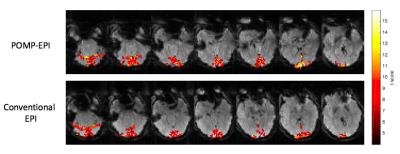
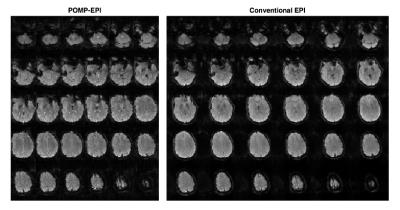
Raw POMP-EPI images (Fig5) were reconstructed and rearranged for comparison with conventional-EPI images. Due to the long readout, which can be addressed in future studies with partial-Fourier, there is slightly more distortion in the POMP-EPI images. The t-score maps overlaid on the raw images show more activation with the POMP-EPI scans, which may be attributed to the higher acquisition efficiency; under the same scan time of 256s, almost 2 (256/143=1.8) times more time frames can be collected with the POMP-EPI sequence. POMP-EPI may have significant impact for future research and clinical practice due to its ease of implementation (no parallel-imaging required) and higher temporal resolution.Acknowledgements
NIH P41 EB01589References
1. Glover, G. H., Phase-offset multiplanar (POMP) volume imaging: a new technique. J Magn Reson Imaging 1991, 1 (4), 457-61.
2. Souza, S. P.; Szumowski, J.; Dumoulin, C. L.; Plewes, D. P.; Glover, G., SIMA: simultaneous multislice acquisition of MR images by Hadamard-encoded excitation. J Comput Assist Tomogr 1988, 12 (6), 1026-30.
3. Griswold, M. A.; Jakob, P. M.; Heidemann, R. M.; Nittka, M.; Jellus, V.; Wang, J.; Kiefer, B.; Haase, A., Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med 2002, 47 (6), 1202-10.
4. Pruessmann, K. P.; Weiger, M.; Scheidegger, M. B.; Boesiger, P., SENSE: sensitivity encoding for fast MRI. Magn Reson Med 1999, 42 (5), 952-62.
5. Breuer, F. A.; Blaimer, M.; Heidemann, R. M.; Mueller, M. F.; Griswold, M. A.; Jakob, P. M., Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging. Magn Reson Med 2005, 53 (3), 684-91.
6. Setsompop, K.; Gagoski, B. A.; Polimeni, J. R.; Witzel, T.; Wedeen, V. J.; Wald, L. L., Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med 2012, 67 (5), 1210-24.
Figures