1627
Local B0 temporal instability detected using a modified Multi-Echo GRE-EPI sequence with Flipped-Blips (MEPI-FB): application to fMRI with visual presentation of faces1Department of Physics, University of Pisa, Pisa, Italy, 2Imago7 Research Center, Pisa, Italy, 3GE Healthcare, Pisa, Italy, 4GE Healthcare, Orsay, France, 5GE Global Research, Munich, Germany, 6IRCCS Stella Maris, Pisa, Italy
Synopsis
Field-mapping techniques using a reference scan allow accurate distortion correction of EPI data. However, due to the strong B0 dependency with head motion and other physiological artefacts in fMRI acquisitions, the reference field-map does not adequately correct for dynamic non-linear changes in image intensity. In this work we show the feasibility of a method to map temporal variations of the B0-field at 7 Tesla, using a high-resolution Multi-echo EPI sequence, acquiring three echo-modules with reverse phase-encoding direction on the second echo. The robustness of the method was tested during an fMRI acquisition with visual presentation of faces.
Purpose
In addition to static field inhomogeneities that cause severe geometric distortions, EPI-based fMRI time-series are sensitive to either task-related local magnetic field changes, or unwanted inhomogeneities related to head motion and physiological effects1-4. The latter generate local non-linear variations in image intensity, which a static field-mapping approach is unable to correct5-8. To track the field changes in time, multi-echo EPI approaches have been developed9,10. However, these were acquired at low-spatial resolution and the dynamic B0 corrections added unwanted variance to the BOLD data. This study demonstrates the feasibility of a modified Multi-echo EPI with Flipped-Blips (MEPI-FB) approach to map the B0-field at each TR, and subsequently apply dynamic Distortion Correction (DC) in high-resolution functional signal at 7Tesla.Acquisition
Two healthy subjects were scanned on a 7Tesla MRI scanner (GE Healthcare, USA) using a 32-channel head coil (Nova Medical, Wilmington, USA). A multi-echo GRE-EPI sequence with three echo-modules was modified to reverse the PE direction on the second module (Echo2): first (Echo1) and third (Echo3) modules were acquired with Anterior-Posterior direction (A->P), while Echo2 was acquired in P->A. Scanning parameters of the MEPI-FB sequence were: TR=3s, TEs =20, 53.7, 87.4ms, FA=62º, matrix=192x192, FoV=20.0cm, slice-thickness=1.5cm, echo-spacing=728μs, gap=1.0cm, #slices=20, ASSET=3. Slices were prescribed axially in subject 1 and obliquely in subject 2, covering the occipital and temporal lobes. Subjects were scanned during a block-design (A-r-B-r-…) visual fMRI experiment: blocks A and B displayed either famous faces or unknown faces, respectively, to the subjects for 18s, and they were separated by 15s of blank gray screen. In total, 5’30’’ of functional data were acquired, with 12’’ of dummy scans at the beginning. Respiration and cardiac waveforms were recorded.
A distortion-free conventional 2D-GRE sequence with the same prescription as the functional scans was acquired for comparison of brain geometry. In addition, for Subject 1, a conventional 3D B0-map was acquired for comparison.11 For Subject 2, a resting-state MEPI-FB was scanned (Run#2) with identical parameters as before.
Analysis
To estimate the ΔB0 maps each TR (ΔB0(t)), a virtual image, v-Echo2, was calculated from Echo1 and Echo3 modules9 having the same contrast as Echo2 but with A->P PE. Smoothing (FWHM=1.56mm) was applied to Echo2 and v-Echo2 for noise reduction. Following this, the reversed-PE Echo2-pair was used to determine the ΔB0 with a 3D B0-field mapping method7, implemented in topup from FSL12. The BOLD module, Echo1, was unwarped with the corresponding estimated time-point ΔB0 map, resulting in a dynamic DC (dy-DC) time-series, which was subsequently motion corrected. For comparison, a static DC (st-DC) was processed after motion correction by unwarping all Echo1 volumes with the field-map of the first volume.
To assess high-frequency temporal variability of ΔB0, the voxel-by-voxel temporal standard deviation of the first-order detrended distortion fields was calculated (t-SD(ΔB0)). In addition, a general-linear-model (GLM) was used to test the correlation between physiological data (respiration and cardiac recordings, and motion parameters) and the ΔB0 time-series.
In the fMRI runs and within the BOLD module, after FILM prewhitening12, the following GLMs were computed: (1) a dy-DC GLM that included the task regressor, respiration and cardiac waveforms, motion parameters and the ΔB0(t) as explanatory variables (EV); (2) a st-DC GLM, with identical EVs but excluding the ΔB0(t).
Results
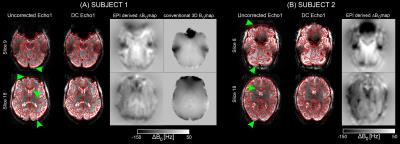
The effect of the proposed DC using long TEs is shown for two subjects in inferior and superior brain slices in Figure 1. Regions with visible geometrical distortions, indicated by green arrowheads, were successfully unwarped to match the distortion-free GRE image (red outline). A good match was found between field-maps obtained with the EPI and the conventional approach11.
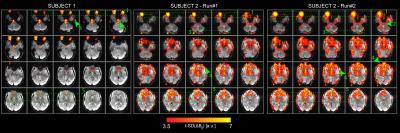
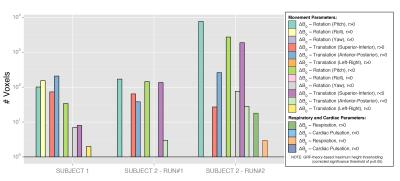
t-SD(ΔB0) maps revealed large temporal variability in regions of the eyes, midbrain, ventricles, vessels and skull (Figure 2). Subject 2 had a maximum head-displacement of 1.69mm (Run#1) and 1.7mm (Run#2) compared to 0.83mm in Subject 1, which explains the average differences in the t-SD(ΔB0) maps. In both subjects motion parameters correlated with ΔB0(t) (Figure 3).
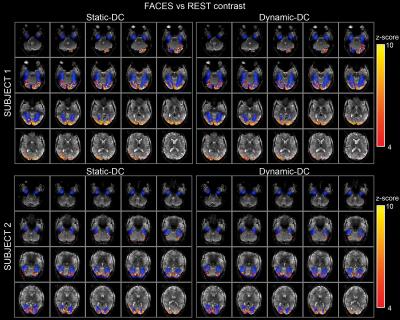
Z-statistical maps originated from the st-DC and dy-DC corrections in both subjects showed strong localized face responsive activations in the fusiform cortex (Figure 4).
Discussion and Conclusion
While realignment can correct for bulk motion after acquisition, significant residual effects often remain13. With a high-resolution MEPI technique at 7 Tesla, we provide a methodology for the detection of multiple movement-by-susceptibility physiological interactions that change brain geometry dynamically in time. In addition to the BOLD module, the two longer-TE modules proved efficient in the correction of geometrical distortions. The dy-DC time-series showed strong localized functional responses as with a st-DC, but further investigation needs to be done to understand the nature of the regressed signal.Acknowledgements
This work was supported by the Initial Training Network, HiMR, funded by the FP7 Marie Curie Actions of the European Commission (FP7-PEOPLE-2012-ITN-316716).References
1. Jezzard P, Clare S. Sources of distortion in functional MRI data. Hum. Brain Mapp. 1999;8:80-85.
2. Zahneisen B, Asslaender J, LeVan P, et al. Quantification and correction of respiration induced dynamic field map changes in fMRI using 3D single shot techniques. Magn. Reson. Med. 2014;71:1093-1102.
3. Zeller M, Kraus P, Mueller A, et al. Respiration impacts phase difference-based field maps in echo planar imaging. Magn. Reson. Med. 2013;72:446-451.
4. Dymerska B, Poser B A, Barth M, et al. A method for the dynamic correction of B0-related distortions in single-echo EPI at 7 T. Neuroimage. 2016.
5. Chen N K, Wyrwicz A M. Correction for EPI distortion using multi-echo gradient echo imaging. Magn. Reson. Med. 1999;41:1206–1213.
6. Zeng H, Constable R T. Image distortion correction in EPI: Comparison of field mapping with point spread function mapping. Magn. Reson. Med. 2002;48(1):137-146.
7. Andersson J, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20(2):870-888.
8. Morgan P S, Bowtell R W, McIntyre D, et al. Correction of spatial distortion in EPI due to Inhomogeneous static magnetic fields using the reversed gradient method. JMRI. 2004;19:499-507.
9. Weiskopf N, Klose U, Birbaumer N, et al. Single-shot compensation of image distortions and BOLD contrast optimization using multi-echo EPI for real-time fMRI. Neuroimage. 2005;24:1068-1079.
10. Hutton C, Bork A, Josephs O, et al. Image distortion correction in fMRI: a quantitative evaluation. Neuroimage. 2002;16:217-240.
11. Jezzard P, Balaban R. Correction for geometric distortion in Echo-Planar Images from B0 field variations. MRM. 1995;34:65-73.
12. Smith S M, Jenkinson M, Woolrich M W, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(S1):208-219.
13. Wu D H, Lewin J S, Duerk J L. Inadequacy of motion correction algorithms in functional MRI: Role of susceptibility-induced artifacts. JMRI. 1997;7:365-370.
Figures