1624
Dual-Echo Cardiac Gating Spiral Sequence for Cervical Spine fMRI1Stanford University, Stanford, CA, United States
Synopsis
We optimized a spiral sequence for dual-echo cardiac gating applied to cervical spine fMRI. Dual-echo obviates dependency on variable initial longitudinal magnetization that would, otherwise, require T1 correction. Spiral acquisition allows short echo time and readout duration which maximizes SNR and BOLD contrast. Our technique improves tSNR and fMRI activation when compared with ungated sequence.
Introduction
Functional Magnetic Resonance Imaging (fMRI) of the cervical spine is challenging partly due to cardiac-related physiological noise. Cardiac influence causes vascular pulsation noise that may obscure spinal cord BOLD (blood-oxygen-level-dependent) response since main arteries and veins run along the cord and adjacent to grey matter.1 Also, cardiac pulsation induces pulsatile movement in cerebrospinal fluid causing non-rigid spinal cord motion and signal variation.2 It is possible to minimize cardiac-induced noise by cardiac-gated acquisition; each image volume being acquired synchronized to heart rate instead of a fixed repetition time $$$TR$$$. The first echo, of a dual-echo acquisition, can eliminate variable initial longitudinal magnetization in cardiac gating that would, otherwise, require correction by precise knowledge of pixel-wise longitudinal relaxation time T1 of imaged anatomy.3,4 Here, we improve this dual-echo technique to detect activation in cervical spine by optimizing echo times $$$TE$$$ and minimizing readout time by means of a custom spiral sequence5. Using the proposed techniques, we increase temporal-SNR (tSNR) and can detect more activation in cervical spine than a non-cardiac gating technique.Methods
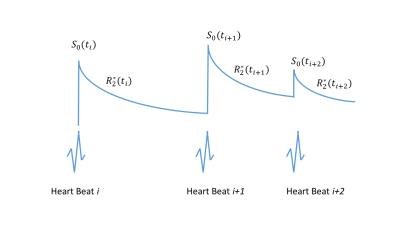
In a cardiac-gated dual-echo sequence (Fig.1), amount of initial transverse magnetization $$$S_0$$$ is different for each image volume because each is acquired synchronized to heart rate ($$$TR$$$ is not fixed, Fig.2).
$$\,\,\,S_1(t,i)| _{t={TE}_1}=S_0(t_i)e^{-{TE}_1 R_2^*(t_i)}\,\,\,\,\,\,\,i=1...N\,\,\,(1)$$
$$\,\,\,S_2(t,i)| _{t={TE}_2}=S_0(t_i)e^{-{TE}_2 R_2^*(t_i)}\,\,\,\,\,\,\,i=1...N\,\,\,(2)$$
$$S_2(i)/S_1(i)=e^{-\triangle{TE}_1 R_2^*(t_i)}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,i=1...N\,\,\,(3)$$
Equations (1) and (2) represent readout magnetization signal intensity at two $$$TE$$$ within the same cardiac acquisition period (collected for $$$N$$$ cardiac periods). Unknown initial signal intensity $$$S_0(t_i)$$$ can be eliminated by dividing the second echo readout by the first (Eqn. 3), which results in a dependence only on $$$R_2^*$$$.
Experiment
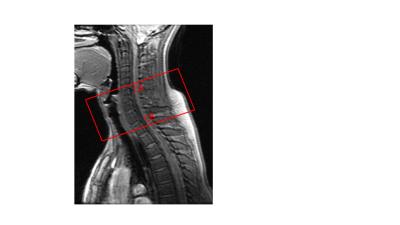
To demonstrate, we gathered cervical spinal data using a cardiac-gated dual-echo spiral-out sequence on a healthy volunteer. Vertebral levels between C4 and C8 were imaged (Fig. 3). Scanning parameters are: $$${TE}_1$$$=5ms, $$${TE}_2$$$=35ms, matrix size=64 (two shots), FOV = 16cm, slice thickness = 4mm, gap=1mm, #slices=14. A custom-built MRI-compatible pneumatically driven finger-moving device was engaged for passive motor task: velcro straps secure volunteer’s hands to the pneumatic device which moves each finger of each hand individually and randomly during on-blocks. Two 128-volume scans were collected: one with cardiac gate every two heart beats, and one with fixed $$$TR$$$=2s. Passive task was a block design alternating between on and off blocks of 15s each. A pulse oximeter was attached to the volunteer’s toe for gating.Analysis
For cardiac-gated data, ratio images were first calculated according to Eqn.3 (with masking to avoid division by zero), then the time-series was resampled to a fixed interval of 2s. To determine activation, time-series of each voxel was correlated with sine and cosine functions at the fundamental task frequency. (Higher-order harmonics were not modeled because they were strongly attenuated by hemodynamic filtering and because of relatively short block periods.) tSNR images were calculated for both cardiac-gated and ungated data for comparison.Results
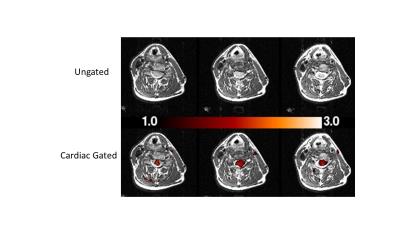
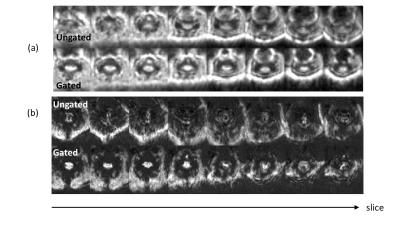
Figure 4 shows activation in three slices: from ungated and cardiac-gated data, each overlaid on anatomical images. Activation was reliably detected using our optimized cardiac gating method but not with the ungated method. Figure 5a shows eight slices (after dividing second echo by the first) and their corresponding tSNR images (Fig.5b). Cardiac-gating significantly improves tSNR over ungated acquisition.Discussion
The basic premise of dual-echo cardiac gating is: division of two echos in order to eliminate unknown initial magnetization $$$S_0$$$; thus, avoidance of imperfect T1 effect correction. We have optimized this technique, beyond earlier attempts,3,4 by employing a spiral sequence instead of EPI. Since the spiral begins traversal of k-space starting from its center, $$${TE}_1$$$ can be set to a much shorter value than that of EPI (5ms vs 21ms). Shorter $$${TE}_1$$$ and $$${TE}_2$$$ maximize SNR and minimize division error, while keeping $$$\triangle{TE}$$$ equal to $$$T^*_2$$$ is optimal for activation detection. We set echo times for optimal BOLD contrast at 3T ($$${TE}_1$$$=5ms, $$${TE}_2$$$=35ms, $$$\triangle{TE}$$$=30ms). Because spinal cord is highly inhomogeneous, spiral's relatively short readout duration (when compared with EPI) minimizes signal dropout and distortion.5
Cardiac-gating acquisition is inherently less time-efficient, than acquisition at a fixed $$$TR$$$, simply because some time is spent waiting for the next heart beat. These relatively longer periods between each acquisition, on the other hand, allow longitudinal signal to recover more; hence, increasing SNR. We also showed that there is further advantage, due to elimination of motion-related signal loss in these small cervical structures, resulting in increased tSNR; thus, improving image quality.
Acknowledgements
GE Healthcare and NIH P41-EB0015891References
1. Cohen-Adad J, Gauthier CJ, Brooks JCW, Slessarev M, Han J, Fisher JA, Rossignol S, Hoge D. BOLD signal responses to controlled hypercapnia in human spinal cord. NeuroImage 2010; 50:1074–1084.
2. O’Connell JEA. The Vascular Factor In Intracranial Pressure and the Maintenance of the Cerebrospinal Fluid Circulation. Brain 2010; 66:204–228.
3. Zhang WT, Mainero C, Kumar A, Wiggins CJ, Benner T, Purdon PL, Bolar DS, Kwong KK, Sorensen AG. Strategies for improving the detection of fMRI activation in trigeminal pathways with cardiac gating. Neuroimage. 2006;31(4):1506-12.
4. Beissner F, Baudrexel S, Volz S, Deichmann R, Dual-echo EPI for non-equilibrium fMRI - implications of different echo combinations and masking procedures. Neuroimage 2010; 52(2):524-31.
5. Glover GH. Spiral imaging in fMRI. Neuroimage 2012;62(2):706-12.
Figures