1617
Assessment of the Myotendinous Junction After Injury in Elite Athletes Using 3D Ultrashort Echo Time Magnetization Transfer (UTE-MT) Imaging and Modeling1University of California, San Diego, San Diego, CA, United States, 2Orthopedic Surgery, Scripps Clinic, San Diego, California, USA, 3Orthopedic Surgery, University of California, San Diego, United States, 4Radiology Service, VA San Diego Healthcare System, San Diego, United States
Synopsis
Myotendinous injuries are very common in sports, most often affecting the lower extremities. Both clinical examination and MRI have been widely used for the diagnosis and prognosis of acute myotendinous injuries. However, current paradigms remain insufficient as re-injury rates are as high as 34%. For elite athletes, the balance between rehabilitation time and risk of re-injury after return to play can be extremely challenging. Using conventional MRI techniques, clinical interpretation focuses on the evaluation of edema, fluid, and hemorrhage rather than the assessment of the injured/healing components of the myotendinous junction. Furthermore, immature and mature fibrous scar tissue as well as native tendon have very short T2 values and are “invisible” with clinical MRI, thus precluding their distinction and assessment. A technique to quantitatively characterize macromolecules in injured/healing myotendinous junctions could be particularly useful in recovering athletes.
Introduction
Myotendinous injuries are very common in sports, most often affecting the lower extremities. Both clinical examination and MRI have been widely used for the diagnosis and prognosis of acute myotendinous injuries. However, current paradigms remain insufficient as re-injury rates are as high as 34%. For elite athletes, the balance between rehabilitation time and risk of re-injury after return to play can be extremely challenging. Using conventional MRI techniques, clinical interpretation focuses on the evaluation of edema, fluid, and hemorrhage rather than the assessment of the injured/healing components of the myotendinous junction. Furthermore, immature and mature fibrous scar tissue as well as native tendon have very short T2 values and are “invisible” with clinical MRI, thus precluding their distinction and assessment. A technique to quantitatively characterize macromolecules in injured/healing myotendinous junctions could be particularly useful in recovering athletes.Purpose
To utilize the 3D ultrashort echo time magnetization transfer (UTE-MT) technique with two-pool quantitative MT modeling to assess the healing myotendinous junction in elite athletes.Methods
Subjects: Three athletes (2 professional baseball players and an elite soccer player, mean 30 years of age) were imaged after myotendinous junction injuries. Injuries ranged from grades 2-3 and occurred from a range of 2 weeks to 2 years prior to imaging. Protocol: Imaging was performed on a 3T clinical scanner (Signa HDx, GE Healthcare, Milwaukee, WI) using an 8-channel receive-only torso coil. Both lower extremities were imaged, including ipsilateral injured sides and contralateral uninjured sides for comparison as internal controls. In addition to conventional clinical sequences, 3D UTE-MT imaging was performed. The MT preparation consisted of a Fermi shaped RF pulse and two MT powers (300° and 700°) and five MT frequency offsets (2, 5, 10, 20 and 50 kHz) were used. A 3D UTE-Cones sequence1 was used for acquisition with the following parameters: TR=100ms, TE=32μs, FA=7°, FOV=30-33cm, matrix=256×256, slice thickness=5mm, 26 slices. In total 10 different 3D MT datasets were acquired at 2.5 min per dataset. Image Processing and Analysis: Analysis was performed using MATLAB (Mathworks, Natick, MA). Two‐pool UTE‐MT modeling was performed on the datasets using a new rectangular pulse (RP) approximation model with multiple spokes (Nsp) acquired per MT preparation 2, which outperforms the widely used continues wave power equivalent (CWPE) model 3. UTE-MT modeling parameters were generated including macromolecular and water pool fractions, T2 value of the macromolecular pool, and exchange rates from macromolecular to water protons.Results
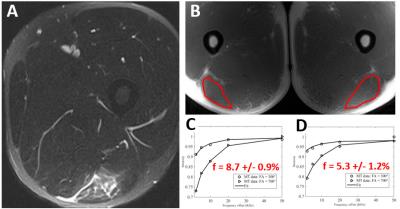
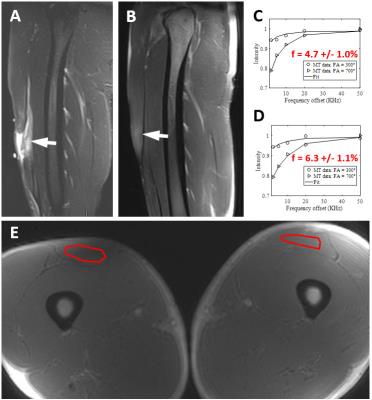
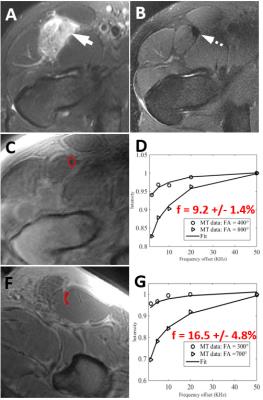
Figure 1 demonstrates images from a professional baseball player with a grade 2 hamstring injury 2 weeks prior. The macromolecular fraction of the injured left biceps femoris muscle measured 5.3%, which was 61% of the value compared to the uninjured side (measuring 8.7%). The patient was able to return to play three weeks after the MRI. Figure 2 demonstrates images from another professional baseball player with a grade 3 quadriceps injury 4 months prior. The macromolecular fraction of the injured left rectus femoris muscle measured 4.7%, which was 75% of the value compared to the uninjured side (measuring 6.3%). The patient was able to return to play one week after the MRI. Figure 3 demonstrates images from an elite soccer player with a grade 3 quadriceps injury 2 years prior. The macromolecular fraction of the healed tendon was 9.2%, which was 55% of the value compared to the uninjured side (measuring 16.5%). This lower value is consistent with what is well known in the literature regarding tendon healing, which is dominated by scar tissue formation with inferior biomechanical properties.Discussion
The UTE-MT technique can be used to assess all of the tissues at the injured/healing myotendinous junction, including muscle, tendon, and fibrous scar. Consistent with previous studies evaluating MT parameters acquired using longer TEs4, range of UTE-MT parameters such as macromolecular fraction depends on particular tissue type and location. Since most MT parameters are magic-angle insensitive2, these variations may represent differences in regional composition and structure.
Despite regional variations in macromolecular fraction, simultaneous imaging of the unaffected, contralateral limb at the same cross-sectional location provides an internal control which can be used for comparison. For healing tissues, our results suggest that macromolecular fraction may not return to baseline values of the contralateral side, even after two years and return to full activity. This is consistent with histological studies which have shown that the majority of tendon and some of muscle healing is characterized by fibrovascular scarring 5,6. The UTE-MT technique may be able to characterize these changes and future studies will be performed to determine the usefulness of these measures in aiding return to play decisions.
Acknowledgements
The authors acknowledge grant support from NIH (1R01 AR062581-01A1, 1 R01 AR068987-01) and VA Clinical Science R&D Service (Merit Award I01CX001388).References
[1] Chen J, Chang EY, Carl M, Ma Y, Shao H, Chen B, Wu Z, Du J. Measurement of bound and pore water T1 relaxation times in cortical bone using three-dimensional ultrashort echo time cones sequences. Magn Reson Med. 2016.
[2] Ma YJ, Shao H, Du J, Chang EY. Ultrashort echo time magnetization transfer (UTE-MT) imaging and modeling: magic angle independent biomarkers of tissue properties. NMR Biomed. 2016 Nov;29(11):1546-1552.
[3] Ramani A, Dalton C, Miller DH, Tofts PS, Barker GJ. Precise estimate of fundamental in-vivo MT parameters in human brain in clinically feasible times. Magn Reson Imaging. 2002;20(10):721–731.
[4] Stanisz GJ, Odrobina EE, Pun J, Escaravage M, Graham SJ, Bronskill MJ, Henkelman RM. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn Reson Med. 2005 Sep;54(3):507-12.
[5] Yang G, Rothrauff BB, Tuan RS. Tendon and ligament regeneration and repair: clinical relevance and developmental paradigm. Birth Defects Res C Embryo Today. 2013;99(3):203-22.
[6] Gharaibeh B, Chun-Lansinger Y, Hagen T, Ingham SJ, Wright V, Fu F, Huard J. Biological approaches to improve skeletal muscle healing after injury and disease. Birth Defects Res C Embryo Today. 2012;96(1):82-94.
Figures