1614
Simultaneous Quantification of Fat Fraction and R2* as measures of Bone Oedema/Adiposity and Structure in Spondyloarthritis1Centre for Medical Imaging, University College London, London, United Kingdom, 2Medical Physics, University College London Hospitals, 3Centre for Adolescent Rheumatology, University College London
Synopsis
Treatment decisions in patients with spondyloarthritis increasingly involve the use of MRI to assess the extent, severity and type of inflammation surrounding the joints. However, conventional spin echo imaging requires subjective interpretation and gives little information about trabecular structure, despite the fact that new bone formation and bone destruction may both occur in spondylorthritis. Here, we describe the use of chemical shift-encoded MRI as a quantitative method for assessing inflammation of the sacroiliac joints. Specifically, we demonstrate the use of proton-density fat fraction and R2* as measures of bone marrow composition and structure, respectively, in both active and chronic inflammation.
Introduction
Treatment decisions in patients with spondyloarthritis increasingly involve the use of magnetic resonance imaging to assess the extent, severity and type of inflammation surrounding the joints 1. ‘Active’ inflammation causes an increase in bone marrow water content (oedema) whilst chronic inflammation may cause an increase in fat content known as fat metaplasia 1,2. Clinically, T2-weighted and T1-weighted spin echo images are used to qualitatively assess active and chronic inflammation respectively 1. However, this approach relies on visual comparison of image intensities, which is highly subjective and depends on the specific sequences and scanner being used. Furthermore, despite the fact that new bone formation and bone destruction may both occur in spondyloarthritis 3, spin echo images provide little information about bone density.
In this study, we describe the use of chemical shift-encoded MRI (CSE-MRI) as a quantitative method for assessing inflammation in the sacroiliac joints. Specifically, we demonstrate the use of proton-density fat fraction (PDFF) and R2* as measures of bone marrow composition and trabecular structure, respectively, in areas of active and chronic inflammation.
Methods
Patients were included in the study if they had a known diagnosis of sacroiliitis or were suspected of having sacroiliitis after assessment by a rheumatologist, and were aged between 12 and 24. Patients with suspected sacroiliitis were treated as controls if the clinical MRI scan and subsequent clinical assessment was negative for inflammation. In addition, eight healthy volunteers had repeat MRI scans of the sacroiliac joints approximately one month apart to assess measurement repeatability.
All patients underwent CSE-MRI of the sacroiliac joints on a 3.0T clinical system (Ingenia, Philips, Amsterdam, Netherlands) with anterior surface and integrated posterior coils using a vendor-supplied Dixon-encoded acquisition for PDFF measurement (Philips mDixon Quant: six-echo 3D-spoiled gradient echo first echo 1.17ms, echo spacing 1.6ms, repetition time 25ms, flip angle 3°). The imaging plane was angled to the sacrum and 40 slices were acquired with a slice thickness of 2mm. PDFF and R2* maps were generated in-line on the scanner. Additionally, all patients underwent conventional T1-weighted and fat-suppressed T2-weighted MRI on a 1.5T system (Avanto, Siemens, Berlin, Germany) on the same day.
In patients with spondyloarthritis, regions-of-interest were placed on areas of bone marrow oedema and/or fat metaplasia, as identified using conventional spin echo images. In controls, regions-of-interest were placed on normal subchondral bone marrow. Differences in fat fraction and R2* between areas of normal bone marrow, oedema and fat metaplasia were compared using a multilevel mixed-effects linear regression model which accounted for clustering effects. Repeatability was assessed using the intraclass correlation coefficient.
Results
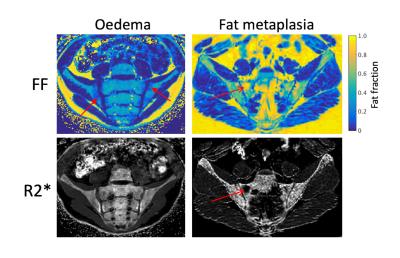
16 subjects were recruited with a median age of 16y6m, of which 8 subjects had evidence of active or chronic sacroiliitis using conventional MRI. The remaining 8 subjects were treated as controls. Example PDFF and R2* maps in patients with active and chronic sacroiliitis are shown in Figure 1. PDFF and R2* measurements are compared by tissue group in Figure 2 and Figure 3.
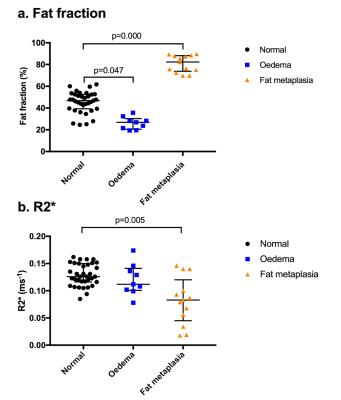
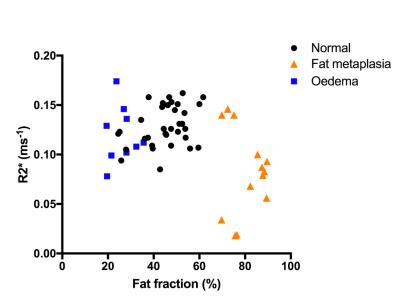
Median PDFF values were 46.8% in normal marrow, 26.9% in bone marrow oedema and 82.3% in fat metaplasia. Compared to normal bone marrow, PDFF measurements were significantly lower in areas of oedema (p=0.047) and were significantly higher in areas of fat metaplasia (p=0.000).
Median R2* values were 0.126ms-1 in normal marrow, 0.112ms-1 in bone marrow oedema and 0.083ms-1 in fat metaplasia. R2* was significantly lower in areas of fat metaplasia (p=0.005) but there was no significant difference in R2* between normal bone and bone marrow oedema (p=0.461).
Eight healthy volunteers were recruited with a median age of 31y0m. The repeatability of both PDFF and R2* measurements in these subjects was excellent, with intraclass correlation coefficients of 0.996 and 0.997 respectively.
Discussion
In this study, we demonstrate significant changes in PDFF in areas of bone marrow oedema and fat metaplasia, suggesting that CSE-MRI could be used as an alternative to conventional techniques for evaluating inflammatory change. CSE-MRI is fast, repeatable and more objective than visual scoring.
In addition, we demonstrate a reduction in R2* in areas of fat metaplasia, which is a new finding. Previous studies indicate an association between bone density and R2* measurements 4,5, and our data suggest that fat metaplasia may therefore be a form of post-inflammatory osteoporosis. R2* measurements could be used to monitor new bone formation and bone destruction in patients with spondyloarthritis.
Conclusion
Chemical shift-encoded MRI could be used as a quantitative method for assessing both active and chronic inflammatory changes in spondyloarthritis. PDFF and R2* measurements could be used to monitor changes in bone marrow composition and structure respectively.Acknowledgements
We are grateful to Hema Chaplin for her help with patient recruitment.References
1. Sieper, J. et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann. Rheum. Dis. 68 Suppl 2, ii1–44 (2009).
2. Maksymowych, W. P., Wichuk, S., Chiowchanwisawakit, P., Lambert, R. G. & Pedersen, S. J. Fat metaplasia and backfill are key intermediaries in the development of sacroiliac joint ankylosis in patients with ankylosing spondylitis. Arthritis Rheumatol. (Hoboken, N.J.) 66, 2958–67 (2014).
3. Neidhart, M. et al. Expression of cathepsin K and matrix metalloproteinase 1 indicate persistent osteodestructive activity in long-standing ankylosing spondylitis. Ann. Rheum. Dis. 68, 1334–1339 (2009).
4. Majumdar, S., Thomasson, D., Shimakawa, A. & Genant, H. K. Quantitation of the susceptibility difference between trabecular bone and bone marrow: Experimental studies. Magn. Reson. Med. 22, 111–127 (1991).
5. Davis, C. A. & Dunham, J. S. The effects of bone on proton NMR relaxation times of surrounding liquids. Invest Radiol 21, 472–477 (1986).
Figures