1612
On the Source of MRI Off-Resonance Observables in Arthroplasty-Induced Metallosis1Radiology, Medical College of Wisconsin, Milwaukee, WI, United States, 2MRI Division, Hospital for Special Surgery, New York, NY, United States, 3Anatomic Pathology and Orthopaedic Surgery, Cleveland Clinic, Cleveland, OH, United States
Synopsis
The deposition of metal particles near total joint replacements has substantial impact on patient morbidity. Current standards of clinical management of patients with symptomatic total hip replacements are highly leveraged on the presence of wear debris in periprosthetic tissues. Our group has recently developed methods to perform off-resonance based identification of metal particle deposits. Here, we provide analysis on the source of tissue-based metal particle Larmor frequency offsets. The results of this analysis provide new insight on the role of off-resonance observables in the progression of symptomatic failed total hip replacements.
Introduction
Our previous work utilized off-resonance mapping techniques to identify and localize regions of suspected metallic debris in the synovial tissues surrounding implanted total hip arthroplasty constructs1,2. We validated our analysis by comparing our in-direct assessment of debris to direct-measures of debris by examination of histologic samples near the implant in patients who underwent revision THA surgery. The results only found a weak correlation between the observed MRI off-resonance pockets and histologic metallosis scores2. The lack of a strong correlation of these metrics was unexpected. A potential confounding factor is that these analyses assumed that deposits of conglomerated particles produce the off-resonance signature that we are observing using MRI methods.
In the present study, we utilize simulations, to show that our prior assumption is not well-founded and we formulate an alternative hypothesis on the source of this off-resonance observed metallosis phenomena in MRI.
Methods
A Monte Carlo simulation was developed to estimate off-resonance signatures within an 8mm (2mm side length) cubic voxel for a variety of potential macroscopic CoCr particle distribution densities with 30um granulation. A magnetic susceptibility of 1000ppm was utilized for the cobalt-chromium debris, which was then passed through a forward-dipole model that predicts MR-observable off-resonance fields 4,5. The simulation was run in Monte-Carlo fashion for 1000 iterations for particle densities ranging from 0.05 to 0.5% by volume. A final off-resonance signature for the voxel was estimated by taking the mean of all off-resonance in regions not occupied by the particles themselves, followed by mean and standard deviations for all simulation in the Monte Carlo analysis.Results
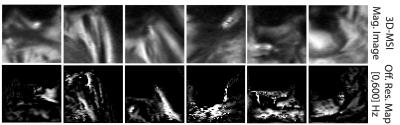
The results of one of the Monte-Carlo simulations, assuming a particle density of 15% by volume, is shown in Figure 1. The average voxel off-resonance values for a range of particle densities results (±SD) is shown in Figure 2. Figure 3 shows 6 cases near suspected metallosis cases in failed total hip replacements that demonstrate large pockets of off-resonance in the suspected regions of tissue disease. These observations utilize methods we have previously presented1,2.Discussion
The results indicate that large conglomerated cobalt-chromium particles themselves cannot be the source of the off-resonance signature that is detected. Six sample cases of patients with a failed THA due to suspected metallosis (Fig. 3) demonstrate large pockets of off-resonance1,2 in the suspected regions of affected tissue. The local off-resonance signature in these regions is 600 Hz to 1.5 kHz and cannot be attributed to the type of conglomerated particles analyzed in our simulation. As a result, a strong correlation between large, histologically observed metal particles and MR-based off-resonance detection should neither be assumed nor expected. An alternative for the source of the off-resonance signature seen in the revision THA patients (Fig. 3) is the presence of locally disassociated soluble cobalt ions. It is well known that cobalt and chromium disassociate through corrosion processes, which is the motivating principle for blood serum ion testing in patients with symptomatic total hip replacements. In patients with corrosion following hip arthroplasty, a Fenton-like reaction occurs, resulting in reactive oxygen species, formation of iron deposits and cell migration, which leads to tissue necrosis and substantial patient morbidity6 Cobalt disassociates into bivalent and trivalent states, which combine to form cobalt-oxides, cobalt-chloride, and other molecules7. We hypothesize that disassociated ionic cobalt, in its paramagnetic compound formulation, could produce the off-resonance distributions we are observing. For example, cobalt-chloride has a paramagnetic susceptibility (0.16 ppm/mM )8 that is larger than that of gadolinium compounds (0.11 ppm/mM)9. Using this number, we can approximate that a concentration of cobalt-chloride at roughly 60mM will yield the 600Hz offsets we are detecting at 1.5T. This is, of course, neglecting other molecular forms of disassociated cobalt ions, such as cobalt-oxides, which have similar orders of magnitude in their paramagnetic magnetic susceptibilities. This simplistic analysis provides a viable explanation connecting MR-based off-resonance observations in regions of likely metal deposition. The clinical impact of this potential biomarker is significant. If disassociated cobalt is the source of observed MR off-resonance signatures, it may provide valuable information on the presence or likelihood of adverse local tissue reactions near hip arthroplasty prior to discernible damage noted on MRI evaluation with artifact reduction sequences such as the 3D multispectral imaging techniques. Future work to elucidate this connection could include mass spectrometry analysis and histomorphometry on tissue samples acquired from revisions surgeries to correlate cobalt compound concentrations, inflammation, and MR-observed off resonance effects.Acknowledgements
This work was supported by NIAMS/NIH (R01-AR064840). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The work was also supported by a grant from the Advancing a Healthier Wisconsin Research and Education ProgramReferences
[1] Koch KM, et al. (2015). A Mechanism for Quantifiable MRI-Based Detection of Cobalt-Chromium Particulate Deposits Near Total Hip Replacements, Proc ISMRM , #310
[2] Koch KM, et al. (2016) Quantitative Off-Resonance-Based Metallosis Assessment Near Total Hip Replacements: Correlating an Imaging Biomarker with Histology Proc ISMRM, #2285
[3] Nawabi et al (2013). MRI Predicts ALVAL and Tissue Damage in Metal-on-Metal Hip Arthroplasty. Clinical Orthopaedics and Related Research, 472(2), 471–481.
[4] Marques, J., & Bowtell, R. (2005). Application of a Fourier-based method for rapid calculation of field inhomogeneity due to spatial variation of magnetic susceptibility. Concepts in Magnetic Resonance Part B Magnetic Resonance Engineering, 25(1), 65–78.
[5] Salomir, R., de Senneville, B., & Moonen, C. (2003). A fast calculation method for magnetic field inhomogeneity due to an arbitrary distribution of bulk susceptibility. Concepts in Magnetic Resonance, 19(1), 26–34.
[6] Gilbert, J. L., Sivan, S., Liu, Y., Kocagöz, S. B., Arnholt, C. M., & Kurtz, S. M. (2014). Direct in vivoinflammatory cell-induced corrosion of CoCrMo alloy orthopedic implant surfaces. Journal of Biomedical Materials Research Part A, 103(1), 211–223.
[7] Simonsen, L. O., Harbak, H., & Bennekou, P. (2012). Cobalt metabolism and toxicology—A brief update. Science of the Total Environment, the, 432(C), 210–215
[8] Landolt-Börnstein, Numerical Data and Functional Relationships in Science and Technology, New Series, III/19, Subvolumes a to i2, Magnetic Properties of Metals, Springer-Verlag, Heidelberg, 1986-1992.
[9] Hijnen, N. M., Elevelt, A., Pikkemaat, J., Bos, C.,Bartels, L. W, & ll, H. G. (2013). The magnetic susceptibility effect of gadolinium-based contrast agents on PRFS-based MR thermometry during thermal interventions. Journal of Therapeutic Ultrasound
Figures