1611
Chemical Shift T1 Quantification with Three-dimensional Ultrashort Echo Time Cones Imaging and IDEAL (3D UTE-Cones-IDEAL) ProcessingXing Lu1,2, Michael Carl3, Yajun Ma1, Yanchun Zhu1, Yinghua Zhao1, Wenhui Yang2, Eric Y Chang1,4, and Jiang Du1
1Department of Radiology, University of California, San Diego, CA, United States, 2Institute of Electrical Engineering, Chinese Academy of Science, Beijing, People's Republic of China, 3GE Healthcare, San Diego, CA, United States, 4Radiology Service, VA San Diego Healthcare System, San Diego, CA, United States
Synopsis
Conventional T1 measurements are subject to errors due to fat contamination and invisibility of the short T2 components. In this study, we aimed to develop 3D UTE with Cones sampling and IDEAL processing (3D UTE-Cones-IDEAL) with variable TRs for robust fat/water separation and more accurate T1 estimation of joint tissues ex vivo and in vivo. The results show that this technique can robustly separate water and fat signal and lead to longer T1s for water-rich tissues (such as muscle, tendon and peripheral nerve), and shorter T1s for fat-rich tissues.
Introduction
Separation of water and fat is important for morphological and quantitative magnetic resonance imaging (MRI). Iterative decomposition of water and fat with echo asymmetry and least squares estimation (IDEAL) is one such approach1-3, and has been used for more accurate T1 measurement by separating fat and water and minimizing partial volume and chemical shift effects4. Furthermore, many tissues such as muscle, peripheral nerve, bone marrow, etc., have a mix of water and fat as well as short and long T2 components. Conventional T1 measurements are subject to errors due to fat contamination and invisibility of the short T2 components. Ultrashort echo time (UTE) sequences have been developed for imaging of both short and long T2 tissues5-7. In this study, we aimed to develop 3D UTE with Cones sampling and IDEAL processing (3D UTE-Cones-IDEAL) with variable TRs for robust fat/water separation and more accurate T1 estimation of joint tissues ex vivo and in vivo using a clinical 3T scanner.Method
An interleaved multi-echo 3D UTE-Cones acquisition scheme was designed for IDEAL imaging on a clinical 3T scanner (Signa HDx, GE Healthcare, Milwaukee, WI). The 3D UTE-Cones-IDEAL technique was applied to both ex vivo human knee joint specimens and in vivo volunteer forearms. A total of five TRs (20, 40, 60, 80, 120 ms) were used for the ex vivo knee joints and five TRs (12.2, 20, 40, 60,100 ms) were used for in vivo forearm study. For each TR, two interleaved three-echo 3D UTE-Cones acquisitions (ex vivo: TEs = 0.032/2.8/5.6/8.4; 0.8/3.6/6.4/9.2 ms; in vivo: TEs = 0.032/2.9/5.8; 0.8/3.7/6.6ms) were performed. For ex vivo scanning, parameters included: flip angle=20°, matrix = 192*192*20, resolution = 0.833*0.833*3 mm, bandwidth = 125 kHz, total scan time is 67 mins. For in vivo scanning, parameters included: flip angle: 25°, matrix = 160*160*10, resolution = 0.625*0.625*3 mm, bandwidth = 62.5 kHz, total scan time = 29 mins. The following signal model was used to separate fat and water with a graph-cut based IDEAL method: $$ \rho=(\rho_{w}+\rho_{f}\sum \alpha^fe^{-i2\pi ft})\cdot e^{-R_2^*t}\cdot e^{-i2\pi f_{s}t} $$ Water and fat signal $$$\rho_{w}$$$ and $$$\rho_{f}$$$ was obtained then passed to the T1 fitting procedure, as well as the total signal, according to the following equation: $$\rho’ = M_0\cdot\frac{1-e^{-TR/T1}}{1-cos(FA)}e^{-TR/T1}+Base $$ Where, $$$\rho'$$$ can be total signal \rho or calculated water and fat signal rw and rf .Results
Figure 1 shows a selected slice of a cadaveric knee joint imaged with 3D UTE-Cones sequences, as well as IDEAL processing of water and fat images. T1 maps based on regular UTE-Cones and UTE-Cones-IDEAL approaches are also depicted. The latter approach provided significantly increased T1 values for the knee joint tissues such as the patellar tendon (3.63% increase) and muscle (17.92% increase), but lower T1 values for bone marrow (14.16% decrease) and subcutaneous fat (32.03% decrease), as shown in Figure 2. Figure 3 shows a selected slice of the forearm of a healthy volunteer using 3D UTE-Cones imaging, as well as IDEAL processing of water and fat images. T1 maps based on regular UTE-Cones and UTE-Cones-IDEAL approaches were also depicted. Again, the latter approach provided significantly increased T1 values for muscle and peripheral nerves in the forearm. Figure 4 shows T1 fitting from IDEAL and non-IDEAL acquisitions for muscle, peripheral nerve, bone marrow and subcutaneous fat. Similar to the ex vivo results, muscle (14.12% increase) and peripheral nerve (7.19% increase) showed significantly increased T1s with the 3D UTE-Cones-IDEAL approach, while bone marrow (7.57% decrease) and subcutaneous fat (15.18% decrease) showed significantly reduced T1 values.Discussion and Conclusion
The 3D UTE-Cones-IDEAL technique can robustly separate water and fat signal, thus providing more accurate T1 estimation for water-rich tissues such as patellar tendon and muscle, and fat-rich tissues such as bone marrow and subcutaneous fat. Since fat has much shorter T1 than water, robust separation of water and fat leads to longer T1s for water-rich tissues (such as muscle and peripheral nerve), and shorter T1s for fat-rich tissues (such as bone marrow and subcutaneous fat), as shown in Figures 2 and 4. Flip angle error was not considered in this technique, however the combination of 3D UTE-Cones-IDEAL with dual-TR acquisition can potentially correct errors associated with fat contamination and B1 inhomogeneity8,9 and will be investigated in future studies. A major limitation is the long scan time, which may be resolved by optimizing the choice of TRs (currently a broad range of TRs from ~10 to 100 ms were used), as well as utilization of advanced acceleration techniques including parallel imaging and compressed sensing reconstruction.Acknowledgements
The authors acknowledge grant funding from the NIH (R01AR062581-01A1 and R01AR068987-01), VA Clinical Science R&D Service (Merit Award I01CX001388), National Natural Science Foundation of China (NSFC 51607169) and Chinese Scholarship Council Grant (CSC 201504910174). We acknowledge the use of the Fat-Water Toolbox (http://ismrm.org/workshops/FatWater12/data.htm).References
1. Hernando D, Kellman P, Haldar JP, Liang ZP. Robust water/fat separation in the presence of large field inhomogeneities using a graph cut algorithm. Magn Reson Med. 2010 Jan;63(1):79-90. 2. Kijowski R, Woods MA, Lee KS, et al. Improved fat suppression using multipeak reconstruction for IDEAL chemical shift fat-water separation: application with fast spin echo imaging. J Magn Reson Imaging 2009;29(2):436-442. 3. Wang K, Du J. K-space water-fat decomposition with T2* estimation and multifrequency fat spectrum modeling for ultrashort echo time imaging. JMRI. 4. Rakow-Penner R, Daniel B, Yu H, Sawyer-Glover A, Glover GH. Relaxation times of breast tissue at 1.5T and 3T measured using IDEAL. J Magn Reson Imaging 2006; 23:87-91. 5. Robson MD, Gatehouse PD, Bydder M, Bydder GM. Magnetic resonance: an introduction to ultrashort TE (UTE) imaging. J Comput Assist Tomogr 2003;27(6):825-846. 6. Carl M, Bydder GM, Du J. UTE imaging with simultaneous water and fat signal suppression using a time-efficient multispoke inversion recovery pulse sequence. Magn Reson Med 2016; 76:577-582. 7. Chen J, Carl M, Ma Y, Shao H, Lv X, Chen BM, Chang EY, Wu Z, Du J. Fast volumetric imaging of bound and pore water in cortical bone using three-dimensional ultrashort-TE (UTE) and inversion recovery UTE sequences. NMR Biomed 2016;doi:10.1002/nbm.3579. 8. Yarnykh VL. Optimal radiofrequency and gradient spoiling for improved accuracy of T1 and B1 measurements using fast steadystate techniques. Magn Reson Med 2010;63:1610–1626. 9. Han M, Larson P, Krug R, Rieke V. Actual flip angle imaging to improve T1 measurement for short T2 tissues. In: Proceedings of ISMRM 23rd Annual Meeting, Toronto, Canada, June 2015. P501.Figures
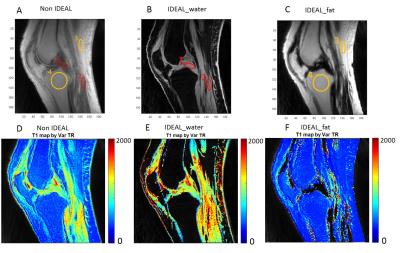
Figure1.3D UTE-Cones imaging of the knee
joint without and with IDEAL processing, as well as the corresponding T1
mapping. The UTE-Cones-IDEAL processing tends to increase T1s for water and
reduce T1s for fat. ROI 1, 2, 3, 4 are selected both in the non-IDEAL image and
IDEAL images accordingly as shown in figure A, B and C.
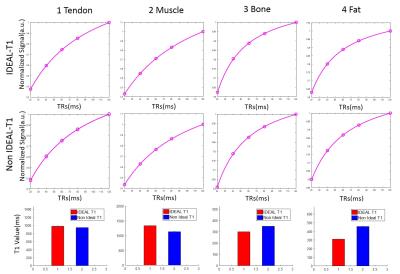
Figure 2. ROI T1 fitting for patellar tendon, muscle, marrow fat
and subcutaneous fat in the knee joint with (1st row) and without (2nd
row) IDEAL processing, and the corresponding T1 values. IDEAL processing provided
significantly increased T1 values for the knee joint tissues such as the
patellar tendon (3.63% increase) and muscle (17.92% increase), but lower T1
values for bone marrow (14.16% decrease) and subcutaneous fat (32.03%
decrease).
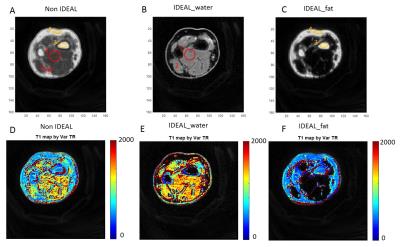
Figure 3. 3D UTE-Cones imaging of the forearm of a 35 age healthy
volunteer without and with IDEAL processing, as well as the corresponding T1
mapping. The UTE-Cones-IDEAL processing increased T1s for water and reduced T1s
for fat. ROI 1, 2, 3, 4 are selected both in the non-IDEAL image and IDEAL
images accordingly as shown in figure A, B and C.
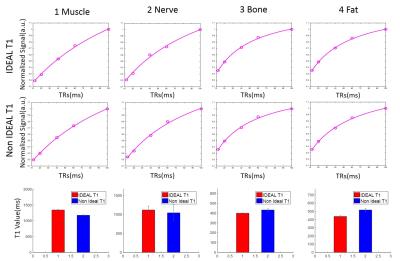
Figure 4. ROI T1 fitting for muscle, peripheral nerve, marrow fat
and subcutaneous fat in the forearm with (1st row) and without (2nd
row) IDEAL processing, and the corresponding T1 values. IDEAL processing provided
significantly increased T1 values for muscle (14.12% increase) and peripheral
nerve (7.19% increase), but lower T1 values for bone marrow (7.57% decrease)
and subcutaneous fat (15.18% decrease).