1593
Quantitative Mapping of the Ischemic Femoral Head in a Piglet Model of Legg-Calve-Perthes Disease1Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 2Veterinary Population Medicine, University of Minnesota, St. Paul, MN, United States, 3Texas Scottish Rite Hospital, Dallas, TX, United States, 4Orthopaedic Surgery, UT Southwestern, Dallas, TX, United States, 5Radiology, University of Minnesota, Minneapolis, MN, United States
Synopsis
This study investigated the sensitivities of T1, T2, continuous-wave T1ρ, adiabatic T1ρ, and RAFF relaxation times to ischemia-induced necrosis and subsequent repair of the developing femoral head in a piglet model of Legg-Calve-Perthes disease (LCPD), a disabling childhood hip disorder. Quantitative maps of ischemic and control femoral heads acquired ex vivo at 9.4T MRI were compared numerically and validated with histology. Our findings reveal that the relaxation times provide complementary information on the status of the pathological hip, which can potentially address a clinical need for diagnostic imaging tools to assess the early stages of LCPD.
Purpose
Legg-Calve-Perthes disease (LCPD) is a childhood hip disorder caused by ischemic injury to the developing femoral head and can lead to severe joint deformity and fragmentation.1 The pathogenesis of LCPD is complex, where restriction of the femoral head blood supply results in bone, marrow, and cartilage necrosis, bone resorption, infiltration of fibrovascular tissue, and eventually reparative revascularization and new bone formation. If the healing is insufficient and loading is maintained, then severe deformation and fragmentation can result. Current radiological assessments of LCPD use x-ray and traditional MRI to determine the degree of femoral head deformity or fragmentation. However, these techniques cannot reliably assess the ischemic damage and subsequent repair prior to the development of irreversible changes. About 50% of patients with LCPD present clinically with hip pain before irreversible damage occurs, and evidence has shown that early interventions can improve patient outcomes.2,3 Thus, new diagnostic imaging tools are needed to guide clinical management of early-stage LCPD. The purpose of this work was to investigate whether quantitative mapping techniques (T1, T2, continuous-wave (cw) T1ρ, adiabatic T1ρ, and RAFF 4,5) are sensitive to ischemia-induced changes to the femoral head in an animal model of LCPD.Methods
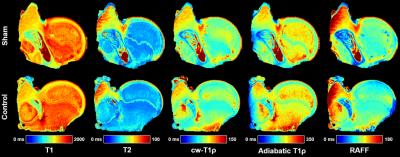
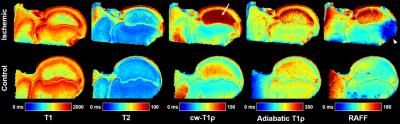
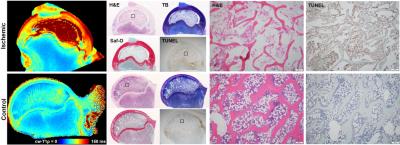
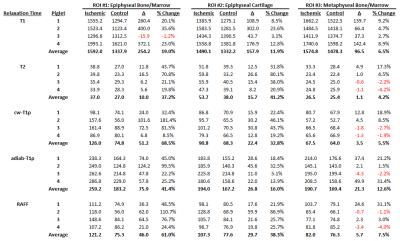
The best available animal model to study LCPD is a piglet model, for which a ligature is placed about the femoral neck and the ligamentum teres is severed to acutely restrict blood supply to the femoral head.1,6 For this study, four 6-week-old male piglets underwent surgery to induce ischemia on one hip. This developmental age is comparable to that of a 5-year-old child, which is a typical age of LCPD onset. A fifth 6-week-old piglet also underwent surgery without placement of a restrictive ligature and thus served as a sham control. The piglets were sacrificed four weeks after surgery, and the operated and unaltered contralateral control femoral heads were harvested and frozen. For imaging, the specimens were thawed, placed in Fomblin, and imaged using a Varian 9.4T preclinical MRI scanner and millipede RF coil. High-spatial-resolution quantitative maps were acquired through the center of the femoral head using a 2D FSE sequence and different magnetization preparation blocks. Typical imaging parameters common to all maps were: FOV=40×40 mm2; matrix=256×256 (yielding 0.16×0.16 mm2 resolution); slice thickness=1.0 mm; TR/TEeff=5000/5.0 ms; ETL=8; and BW=132 kHz. Preparation block parameters included: T1 TI=200, 500, 800, 1100, 1400, 3000 ms; T2 TE=4, 20, 40, 60, 80, 100 ms; cw-T1ρ B1SL=500 Hz and TSL=0, 24, 48, 96, 192 ms; adiabatic T1ρ B1SL,max=2500 Hz and TSL=0, 24, 48, 72, 96, 144 ms; and RAFF B1SL,max=625 Hz and TSL=0, 36.2, 72.4, 108.6, 144.8 ms with two phase-cycled acquisitions. Immediately after imaging, the specimens were bisected along the imaging plane and fixed in 10% neutral buffered formalin. Two of the pairs were then processed for histology along the coregistered imaging plane and stained using H&E, TB, safranin-O, and TUNEL. Quantitative maps were generated using Matlab mono-exponential fitting. Mean relaxation times were calculated for regions-of-interest (ROIs) encompassing the epiphyseal bone/marrow, metaphyseal bone/marrow, and epiphyseal cartilage.Results
The piglet with the sham surgery had very similar relaxation time values for its operated and control hips (Figure 1). Conversely, the four piglets with ischemia had dramatic relaxation time differences between the operated and control hips (Figure 2). ROI relaxation times for all four pairs are shown in Table 1. Histology confirmed widespread epiphyseal bone, marrow, and cartilage necrosis in the operated hips (Figure 3) along with the presence of reparative new bone formation and revascularization. cw-T1ρ and T2 were respectively found to be most sensitive to necrosis of the epiphyseal bone/marrow and epiphyseal cartilage. T2 mapping was most sensitive to regions of new bone formation. The metaphyseal bone/marrow, which does not experience ischemia, served as an internal control and did not large differences between the pairs.Discussion
This study strongly supports that quantitative relaxation time mapping techniques may be an effective tool to assess early-stage LCPD. The finding that cw-T1ρ and RAFF are very sensitive to bone/marrow necrosis is particularly significant, not only due to its importance for evaluation of LCPD but also because these findings are potentially relevant to a variety of bone/marrow disorders including avascular necrosis (AVN) in general. The sensitivity of T2, cw-T1ρ, and RAFF to epiphyseal cartilage necrosis is consistent with a prior study applying these techniques to osteochondritis dissecans, another developmental joint disease with ischemic origins.4 In conclusion, quantitative mapping techniques are sensitive to early-stage pathological changes to the femoral head in LCPD and may help to improve patient care and prevent life-long disability.Acknowledgements
This study was supported in part by the NIH (P41 EB015894) and the W. M. Keck Foundation.References
1. Kim HK, Aruwajoye O, Stetler J, Stall A. Effects of non-weight-bearing on the immature femoral head following ischemic osteonecrosis: an experimental investigation in immature pigs. J Bone Joint Surg Am 2012; 94(24):2228-37.
2. Joseph B, Nair NS, Narasimha Rao KL, Mulpuri K, Varghese G. Optimal timing for containment surgery for Perthes disease. J Pediatr Orthop 2003; 23(5):601-6.
3. Herring JA, Kim HT, Browne R. Legg-Calve-Perthes disease. Part II: Prospective multicenter study of the effect of treatment on outcome. J Bone Joint Surg Am 2004; 86-A(10):2121-34.
4. Wang L, Nissi MJ, Tóth F, Shaver J, Johnson CP, Zhang J, Garwood M, Carlson CS, Ellermann JM. Multiparametric MRI of epiphyseal cartilage necrosis (osteochondrosis) with histological validation in a goat model. PLoS One 2015; 10(10):e0140400.
5. Ellermann J, Ling W, Nissi MJ, Arendt E, Carlson CS, Garwood M, Michaeli S, Mangia S. MRI rotating frame relaxation measurements for articular cartilage assessment. Magn Reson Imaging 2013; 31(9):1537-43.
6. Kim HK, Su PH. Development of flattening and apparent fragmentation following ischemic necrosis of the capital femoral epiphysis in a piglet model. J Bone Joint Surg Am 2002; 84-A(8):1329-34.
Figures