1592
A novel approach to measure tibial component migration by low field markerless magnetic resonance imaging1Centre for Orthopaedic Surgery OCON., Hengelo, Netherlands, 2University of Twente, Faculty of Science and technology, Enschede, Netherlands
Synopsis
This study evaluated if low field MRI is a practical alternative for Roentgen stereophotogrammetric-analysis (RSA) to measure prosthetic migration. This also included determining the optimal registration method for this purpose. The detection of migration on low field MRI was sufficient for clinical use in two of the translation directions and all three rotational directions. Manual registration proved to be the most accurate method for markerless MRI (MMRI) estimation of the migration.
Purpose
Roentgen stereophotogrammetric analysis (RSA) is currently the gold standard to measure early prosthetic migration which can predict aseptic loosening.1–4 The accuracy of RSA varies between 0.05 and 0.5 mm for translation and 0.15⁰ to 1.15⁰ for rotation (95% confidence intervals).5–7 However, RSA has several disadvantages such as the need for invasive markers and harmful X-radiation.8 Therefore, this study evaluates if low field markerless MRI (MMRI) could be a practical radiation free alternative for RSA to measure early prosthetic migration. The purpose was to determine the accuracy of low field MRI for measuring prosthetic migration and to determine and which registration method is most suitable. Low field MRI is used because it is less hampered by susceptibility artifact then high field MRI.Methods
A schematic overview of the research performed to determine the accuracy of low field MMRI to measure prosthetic migration is shown in Figure 1. A 3D model of the tibial component of a knee prosthesis was obtained with a 3D laser scanner (Konica Minolta Vivid 910). A gelatin phantom with a tibial component of a prosthesis implanted in a porcine tibia was scanned on a low field MRI scanner (ESAOTE G-scan 0.25T) in transverse direction with a 2D PD weighted metal artifact reducing sequence PD-XMAR (TE/TR 10/1020 ms, slice thickness 3mm, FOV 180x180x120 mm³, matrix size 224x224). Ten acquisitions were performed; after five the phantom was repositioned without prosthetic motion. The prosthesis and bone were segmented. Afterwards image registration was performed between the segmentations and the 3D models. This was done manually (M), automatically (A) and once by only using the segmentations (W) instead of the 3D models. With a Procrustes algorithm, the translation and rotation of the prosthesis between two scans was calculated with respect to the bone.Results
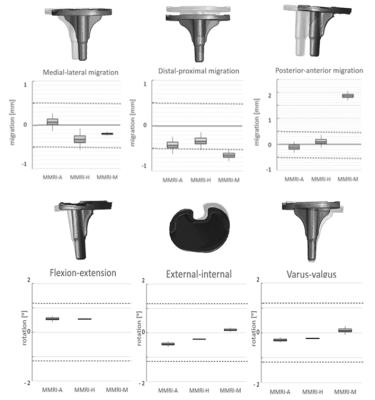
Accuracy levels were considered clinically useful for five of the six degrees of freedom. The boxplots of figure 2 show that MMRI-A and MMR-M are accurately measuring medial-lateral migration within the detection resolution considered accurate enough/comparable to RSA, at -0.14 to 0.27 mm for MMRI-A and at -0.25 to -0.15 mm for MMRI-M. For the posterior anterior migration MMRI-A and MMRI-H are within the area for clinical relevance, at -0.32 to 0.11 mm for MMRI-A and at -0.131 to 0.363 mm for MMRI-H. The overall registration error was the biggest in the distal-proximal direction. Of the three different registration methods, the automatic registration method was most accurate with a mean registration error for translation and rotation of (≤|0.42|mm and ≤ |0.56|⁰).
Discussion
The results illustrate that low field MMRI is a potential alternative for markerless RSA with an obtained accuracy that is comparable to the ranges found in markerless RSA studies7,9. The largest measurement error in our study for all registration methods was found in the distal-proximal direction (y-axis). This can be explained by a trough-plane resolution of 3 mm in the y-axis, which is larger than the in-plane resolution of 0.8x0.8 mm². It is expected that a more detailed segmentation and a better accuracy is acquired if the trough-plane resolution improves,. The manual registration method and the automatic registration method approach the accuracy of RSA the best. The automatic registration algorithm was slightly more accurate. Migration was also estimated without using a 3D model. Although the accuracy of this method was much lower, it could still be an interesting method, since it would allow determination of migration of prostheses with different sizes and shapes. A limitation of this study is that the gelatin used in the phantom is much more homogeneous than human tissue. The heterogeneity of the knee could potentially influence the size of the metal artefact, decreasing the accuracy. For validation of the method it is therefore recommended to evaluate this effect in vivo.Conclusion
Low field MMRI can determine tibial component migration accurately enough for clinical practice in five out of six degrees of freedom. This imaging technique shows potential as a standalone technique to measure early prosthetic migration. Future research should focus on repeated measurements and improving the analysis method.Acknowledgements
No acknowledgement found.References
1. Kärrholm, J., Gill, R. H. S. & Valstar, E. R. The history and future of radiostereometric analysis. Clin. Orthop. Relat. Res. 448, 10–21 (2006).
2. Valstar, E. R., De Jong, F. W., Vrooman, H. a., Rozing, P. M. & Reiber, J. H. C. Model-based Roentgen stereophotogrammetry of orthopaedic implants. J. Biomech. 34, 715–722 (2001).
3. Kärrholm, J. et al. Radiostereometry of hip prostheses. Review of methodology and clinical results. Clin. Orthop. Relat. Res. 94–110 (1997). at <http://europepmc.org/abstract/MED/9372762>
4. Vrooman, H. a. et al. Fast and accurate automated measurements in digitized stereophotogrammetric radiographs. J. Biomech. 31, 491–498 (1998).
5. Valstar, E. R., Nelissen, R. G. H. H., Reiber, J. H. C. & Rozing, P. M. The use of Roentgen stereophotogrammetry to study micromotion of orthopaedic implants. ISPRS J. Photogramm. Remote Sens. 56, 376–389 (2002).
6. Kärrholm, J. Roentgen stereophotogrammetry. Review of orthopedic applications. Acta Orthop. Scand. 60, 491–503 (1989).
7. Seehaus, F., Olender, G. D., Kaptein, B. L., Ostermeier, S. & Hurschler, C. Markerless Roentgen Stereophotogrammetric Analysis for in vivo implant migration measurement using three dimensional surface models to represent bone. J. Biomech. 45, 1540–1545 (2012).
8. Kaptein, B. L., Valstar, E. R., Stoel, B. C., Rozing, P. M. & Reiber, J. H. C. A new model-based RSA method validated using CAD models and models from reversed engineering. J. Biomech. 36, 873–882 (2003).
9. de Bruin, P. W. et al. Image-based RSA: Roentgen stereophotogrammetric analysis based on 2D-3D image registration. J. Biomech. 41, 155–164 (2008).
Figures