1589
MRI Assessment of the Effect of Low-Magnitude Mechanical Stimulation on Postmenopausal Bone Loss1University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Low-magnitude mechanical stimulation (LMMS) has shown great potential as a non-pharmacological intervention for improving bone quality in animal models. However, human trials have yielded less compelling evidence, possibly related to difficulties in maintaining adherence and use of conventional imaging techniques not being able to detect subtle longitudinal changes. Here we investigated the use of high-resolution structural bone MRI in monitoring treatment efficacy of LMMS in postmenopausal women. The data show that baseline bone volume fraction at the distal tibia is associated with the changes observed over one year of active LMMS or placebo treatment.
Purpose
Low bone mass is a key risk factor for osteoporotic fractures and thus a major public health threat for older Americans, particularly for women after menopause. Data in animals have shown that low-magnitude mechanical stimulation (LMMS) treatment is strongly osteogenic1 but the results are less compelling in humans where the effect critically depends on subjects’ adherence.2-5 The goal of this study was to investigate the effect of LMMS on microstructural and mechanical environment of trabecular bone assessed using high-resolution MRI in postmenopausal women.Methods
A double-blinded, randomized, placebo-controlled study involving 100 early postmenopausal women is currently in progress in the authors’ laboratory. Here we report initial data from 26 subjects (age 60 ± 5 years) who have already completed the study. The subjects are randomly assigned to either an active or placebo device involving 10 minutes daily of standing on a platform resembling a bathroom scale (Figure 1). The active device vibrates at 30 Hz and 0.3g (an acceleration corresponding to a displacement of approximately 90 mm, where g = 9.8 m/s2) whereas the placebo device merely emits an equal acoustic signal, which is indistinguishable to the patient from the active device. The distal tibia was imaged on a clinical 3T scanner (Siemens Tim Trio, Erlangen, Germany) using a custom-built 4-channel surface coil and FLASE sequence at 0.137 mm x 0.137 mm x 0.410 mm voxel size6 both at baseline and after one year of intervention. Microstructural bone parameters and whole-section stiffness were computed on the basis of these images using digital topological and finite element analysis, respectively, to test the hypothesis that bone quality assessed in terms of the derived structural and mechanical parameters improves upon treatment. BVF was computed as the average fractional occupancy of bone over the total trabecular volume. Trabecular thickness was computed using the fuzzy distance transform algorithm. Axial stiffness was computed by simulating compressive loading conditions along bone’s axial direction.7Results
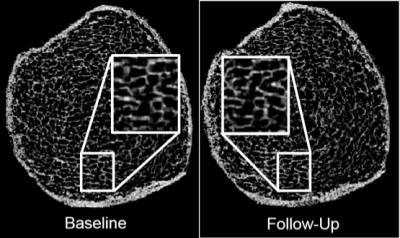
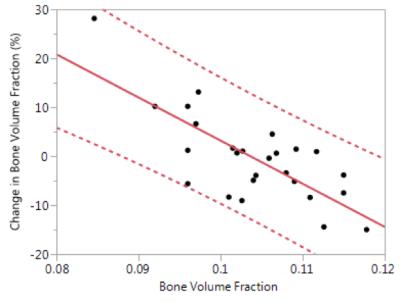
Figure 2 shows a set of representative bone volume fraction (BVF) maps derived from the distal tibia MR images acquired from the same subject at baseline and follow-up. The data suggests that the baseline MRI-derived BVF of trabecular bone was inversely correlated with the fractional change from baseline (Figure 3). Further, changes in axial stiffness were significantly associated with changes in trabecular BVF (R2=0.63) and trabecular thickness (R2=0.71) but not with changes in cortical BVF.Discussion and Conclusion
One notable observation from this dataset was that subjects whose baseline BVF were low had a greater increase in BVF after the completion of the 12-month intervention than subjects whose baseline BVF was already high. The effect of baseline bone quality on the effectiveness of the intervention is potentially significant since BVF is the single largest predictor of the bone’s mechanical behavior estimated by computational biomechanics.6 Since the data has not yet been unblinded, approximately half the subjects were in the placebo group, and thus would not show an effect of LMMS, and thus could continue to decline in BVF as expected following menopause. No definitive conclusions can be drawn from this limited dataset, yet the effect observed is highly statistically significant.Acknowledgements
NIH R01 AR055647 and R01AG038693References
1. Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K. Anabolism. Low mechanical signals strengthen long bones. Nature. 2001 Aug 9;412(6847):603-4.
2. Gilsanz V, Wren TA, Sanchez M, Dorey F, Judex S, Rubin C. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res. 2006 Sep;21(9):1464-74.
3. Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res. 2004 Mar;19(3):343-51.
4. Leonard MB, Shults J, Long J, Baldassano RN, Brown JK, Hommel K, Zemel BS, Mahboubi S, Howard Whitehead K, Herskovitz R, Lee D, Rausch J, Rubin CT. Effect of Low-Magnitude Mechanical Stimuli on Bone Density and Structure in Pediatric Crohn's Disease: A Randomized Placebo-Controlled Trial. J Bone Miner Res. 2016 Jun;31(6):1177-88.
5. Kiel DP, Hannan MT, Barton BA, Bouxsein ML, Sisson E, Lang T, Allaire B, Dewkett D, Carroll D, Magaziner J, Shane E, Leary ET, Zimmerman S, Rubin CT. Low-Magnitude Mechanical Stimulation to Improve Bone Density in Persons of Advanced Age: A Randomized, Placebo-Controlled Trial. J Bone Miner Res. 2015 Jul;30(7):1319-28.
6. Magland JF, Wald MJ, Wehrli FW. Spin-echo micro-MRI of trabecular bone using improved 3D fast large-angle spin-echo (FLASE). Magn Reson Med. 2009 May;61(5):1114-21.
7. Magland JF, Zhang N, Rajapakse CS, Wehrli FW. Computationally-optimized bone mechanical modeling from high-resolution structural images. PLoS One. 2012;7(4):e35525..
8. Rajapakse CS, Magland JF, Wehrli FW. Fast prospective registration of in vivo MR images of trabecular bone microstructure in longitudinal studies. Magn Reson Med. 2008 May;59(5):1120-6.
Figures