1574
Multicomponent 3D-T1ρ and T2 Relaxation Mapping of Skeletal Muscle: In-vivo FeasibilityAzadeh Sharafi1, Gregory Chang 1, and Ravinder Regatte1
1Radiology, New York University, School of Medicine, New York, NY, United States
Synopsis
In this study, we investigated the in-vivo feasibility of multicomponent 3D-T1ρ and T2 relaxation mapping in calf muscle using 3T MRI in clinically feasible scan times on eight healthy volunteers. Our preliminary results demonstrate that the biexponential model better characterized the relaxation behavior in calf muscle and can be used to differentiate between different water compartments associated with macromolecules (collagen and contractile proteins) and extracellular/vascular water in calf muscle.
Purpose
Fibrosis is one of the end-stage processes that affect damaged muscles. In muscle fibrosis, the damaged striated skeletal muscle is replaced mainly by excessive collagen1-2. The change of muscle fiber type will also affect T1ρ and T2 relaxation times. Hence, monitoring of T1ρ and T2 relaxation as non-invasive biomarkers may provide valuable information on muscle fibrosis. Monoexponential mapping and measurement of T1ρ and T2 was investigated in several studies for disease monitoring and studying exercise physiology 3-4. Saab et al. reported multiexponential behavior of T2 relaxation in skeletal muscle. More recently, Araujo et al.2 proposed a method to measure the short T2 component in skeletal muscle (SKM) in the presence of fat using a UTE sequence. Biexponential behavior of T1ρ relaxation has been observed in rat muscle5. The purpose of this work is to evaluate the in-vivo feasibility of biexponential analysis of T1ρ and T2 relaxation times of human calf muscle using 3T MRI in clinically feasible scan times.Methods
IRB-approved T1ρ and T2 imaging were performed on eight healthy volunteers (mean age: 30 ± 4 years) using a 3T MRI scanner (Prisma, Siemens Healthcare, Germany) with a 15-channel Tx/Rx knee coil (QED, Cleveland OH). A 3D Cartesian turbo-Flash sequence was customized to enable T1ρ and T2 imaging with varying spin-lock and echo time respectively. To compensate the effect of field inhomogeneities, the spin-lock pulse was segmented into four parts with alternative phase and a refocusing pulse was applied between two pairs. 3D-T1ρ and T2 weighted images were acquired at 10 different TSLs/TEs: 2/4/6/8/10/15/25/35/45/55ms. The sequence acquisition parameters were as follows: TR/TE 1500ms/4ms, flip angle 8°, matrix size 256×128×64, spin-lock frequency=500Hz, slice thickness = 2ms, FOV = 140mm2. the total acquisition time was decreased to 15 minutes for each 3D data set, using The GRAPPA6 technique with the acceleration factor of 3. T1ρ and T2 relaxation maps were estimated pixel by pixel over five consecutive slices for each volunteer using mono- and biexponential model in five regions of interests (Fig.1): gastrocnemius-medial (GM), gastrocnemius-lateral (GL), soleus (SOL), lateral compartment (LC) and anterior compartment (AC).Results
Biexponential relaxation of T1ρ and T2 were detected in different muscles on all eight healthy volunteers. Mean short/long relaxation components of T1ρ and T2 were 4.6 ± 0.4ms (22.1±2.8% fraction)/33.2±1.8ms (77.9±2.8% fraction) and 4.2±0.3ms (14.6±1.7% fraction)/30.4 ± 0.9ms (85.4±1.7% fraction), respectively (Fig.2). The monoexponential relaxation of T1ρ and T2 were estimated to be 26.9±1.3ms and 24.6±1.0ms, respectively. The statistical analysis showed that the mono-exponential T1ρ was significantly higher (P < 0.001) than T2 across all regions. Moreover, there were significant differences in monoexponential T1ρ/ T2 (P<0.05) between AC and GL as well as AC and SOL muscles. Statistical significance was also observed between short and long components of T1ρ between AC and GL muscles.Discussion and Conclusion
The long relaxation component corresponds mainly to extracellular/vascular water while the short component is thought to attributed to macromolecular (collagen and contractile proteins etc.) and intracellular water compartments. In the absence of proton exchange between two pools, the volume fractions provide reasonable representations of physical volumes of each pool. The preliminary results of our study demonstrate that the biexponential fitting may better distinguish the different water compartments in the calf muscle (Fig. 3). The multicomponent T1ρ and T2 relaxation mapping of skeletal muscle has potential for use in the non-invasive assessment of muscle fibrosis, physiology and monitoring of disease progression.Acknowledgements
This study was supported by NIH grants R01-AR060238, R01 AR067156, and R01 AR068966, and was performed under the rubric of the Center of Advanced Imaging Innovation and Research (CAI2R), a NIBIB Biomedical Technology Resource Center (NIH P41 EB017183).References
1. Carlier, P. G.; Azzabou, N.; de Sousa, P. L.; Hicks, A.; Boisserie, J.-M.; Amadon, A.; Carlier, R.-Y.; Wary, C.; Orlikowski, D.; Laforêt, P., Skeletal muscle quantitative nuclear magnetic resonance imaging follow-up of adult Pompe patients. Journal of inherited metabolic disease 2015, 38 (3), 565-572. 2. CA Araujo, E.; Azzabou, N.; Vignaud, A.; Guillot, G.; Carlier, P., Quantitative ultrashort TE imaging of the short-T2 components in skeletal muscle using an extended echo-subtraction method. Magnetic Resonance in Medicine 2016. 3. Noseworthy, M. D.; Akbari, A. In Mapping Skeletal Muscle Spin-Spin (T2) Relaxation, ISMRM, 2012. 4. Franczak, M. B.; Ulmer, J. L.; Jaradeh, S.; McDaniel, J. D.; Mark, L. P.; Prost, R. W., Spin-Lock Magnetic Resonance Imaging of Muscle in Patients With Autosomal Recessive Limb Girdle Muscular Dystrophy. Journal of Neuroimaging 2000, 10 (2), 73-77. 5. Yuan, J.; Zhao, F.; Chan, Q.; Wang, Y.-X. J., Observation of bi-exponential T1ρ relaxation of in-vivo rat muscles at 3T. Acta Radiologica 2012, 53 (6), 675-681. 6. Griswold, M. A.; Jakob, P. M.; Heidemann, R. M.; Nittka, M.; Jellus, V.; Wang, J.; Kiefer, B.; Haase, A., Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magnetic resonance in medicine 2002, 47 (6), 1202-1210.Figures

Representative T1ρ (first row) and T2
(second row) relaxation maps. (a) Binary maps show the location of mono and biexponential
pixels. (b) Estimated monoexponential T1ρ, and T2 relaxation
maps. (c) Adjusted R2 shows the goodness of monoexponential fit. (d)
Estimated short and (e) long component maps. (f) Adjusted R2 shows
the goodness of fit in biexponential model
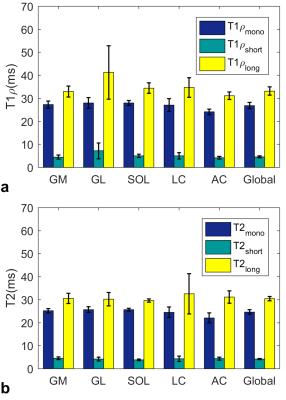
Regional and global mean values of mono and biexponential (a)
T1ρ and (b) T2 relaxation times estimated over eight healthy
volunteers. The mean monoexponential T1ρ/T2 relaxations range
in different regions are [24.1ms, 28ms] / [22ms/25.7ms] with global mean values
of 26.9ms and 24.6ms respectively. The short/long components of were varied
between [4.2ms- 7.22ms] / [31.2ms-41.3ms]. The global short/long T1ρ
relaxation components were 4.5ms and 33.2ms respectively. The biexponential
short/long T2 components varied between [3.8ms- 4.4ms] / [29.60ms-32.52ms]
and the global short/long T2 are 4.2ms and 30.4ms respectively.
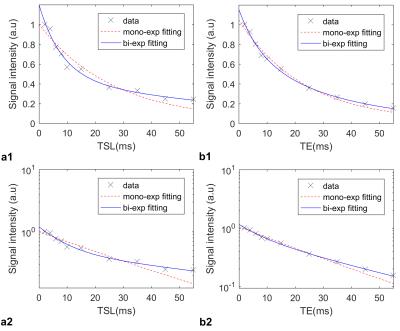
Representative comparison of mono- and
biexponential model for estimating (a) T1ρ and (b) T2
relaxation times. The first row shows the signal intensity in decimal scale
while the data were plotted in logarithmic scale in second row to demonstrate
the dispersion of the data from the straight line (mono exponential). The
biexponential model better represents the relaxation behavior than
monoexponential model.