1573
Targeted modulation of retinoid signaling using polymeric nanoparticles in a mouse model of Amyotrophic Lateral Sclerosis1Neurobiology, Barrow Neurological Institute, Phoenix, AZ, United States, 2Research Imaging, Barrow Neurological Institute, Phoenix, AZ, United States
Synopsis
Retinoid signaling activity in the CNS, mediated by RARβ, directly influences ALS pathology development and progression in vivo and CNS targeted delivery of RARβ agonists can reduce ALS pathology in vivo when delivered systemically via polymeric nanoparticles. In this study the therapeutic affect of adapalene, a RARβ agonist, loaded nanoparticles were examined by measuring quadriceps volume in a SOD1G93A mouse model of ALS.
Introduction
This study examined the role of the retinoic acid (RA) signaling pathway in Amyotrophic Lateral Sclerosis (ALS). RA is a derivative of vitamin A and has been shown to have many important roles in the nervous system such as neuronal development and neurodegeneration. Recent evidence has shown that changes in proteins of the RA signaling pathway are correlated with ALS pathology. Adapalene, an FDA approved RARβ agonist, is currently used for treating acne and cervical cancers. Interestingly, our lab found adapalene to be neuroprotective in a cell culture system. However, adapalene is highly hydrophobic and cannot be delivered systemically. To address this, we developed adapalene loaded nanoparticles to achieve delivery to the CNS. We hypothesize that activation of retinoid signaling will reduce pathology in a transgenic model of ALS.Methods
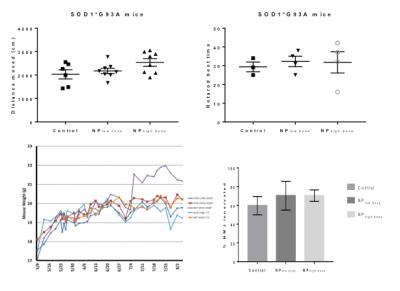
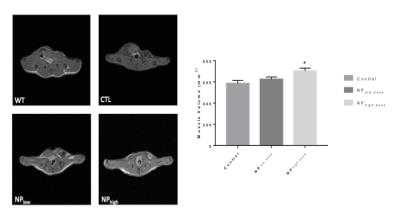
We first designed nanoparticles fabrication by single emulsion to optimize adapalene loading. Adapalene loaded nanoparticles were administered to SOD1G93A mice via lateral tail veins at 8 weeks of age. Motor function was assayed after 4 weeks and 8 weeks of treatment to determine the effect of adapalene on disease progression. Markers of inflammation and neurodegeneration were measured to determine the effect of adapalene on ALS-like pathology. MRI was performed after 8 weeks of treatment on a 7T Bruker Biospec scanner using a 72 mm quadrature volume coil. T1-weighted spin-echo images were acquired covering the entire hind limb (TR=420.6 ms, TE=8.8 ms, 256X256 matrix, 0.176X0.176X1.0 mm voxels, NEX=12). Regions of interest encompassing the quadriceps muscle were used to calculate muscle volume.Results
Administration of Adap-NPs to the brain or via tail vein injection in healthy mice produced upregulation of expected biomarkers, including MAPK signaling in the brain and CRAPB activation in the spinal cord (data not shown). When administered at the maximum deliverable dose (3x/week, 0.25 or 1.25 mg/kg adapalene) to G93A SOD1 ALS mice, Adap-NP significantly slowed disease progression. We observed increased total activity in an open field paradigm, and improved scores on the rotarod behavioral test, decreased weight loss, preservation of the neuromuscular junction and protection against muscle volume loss (Figure 1A). MRI results showed that SOD1G93A mice given the low dose of adapalene had a 6.65% reduction in muscle atrophy while mice given the high dose had a significant (20.0%) reduction in atrophy compared to control (Control 585.93 mm3, NPlow, 624.88 mm3, NPhigh, 703.16 mm3).Discussion and Conclusions
Here we demonstrate that we are able to get activation of the retinoid pathway following systemic administration of nanoparticle-encapsulated adapalene. By using a nanoparticle delivery method we can begin to adequately explore the therapeutic feasibility of modulating RARβ signaling. In parallel studies, we have developed a multiplex tracking approach that enables us the fate of multiple nanoparticles with subcellular resolution in individual subjects; we are using this approach to evaluate brain and spinal cord specific targeting, which we predict will further improve potency of nanoparticle encapsulated adapalene.Acknowledgements
No acknowledgement found.References
1. Kolarcik CL, Bowser R, Retinoid signaling alterations in amyotrophic lateral sclerosis, Am J Neurodeg Dis, 2012; 1(2):130-45
2. Corona J, So PL, Madden M, Absence of retinoids can induce motoneuron disease in the adult rat and a retinoid defect is present in motoneuron disease patients, J Cell Sci, 2002; 115(Pt 24): 4735-41
3. Riancho J, Ruiz-Soto M, Berciano MT, Berciano J, Lafarga M, Neuroprotective Effect of Bexarotene in the SOD1(G93A) Mouse Model of Amyotrophic Lateral Sclerosis, 2015; 1;9:250
Figures