1569
Influence of muscle fiber tension on 31P MRS recovery parameter after intense exercise1Medical Physics Group, Institute of Diagnostic and Interventional Radiology, Jena University Hospital - Friedrich Schiller University Jena, Jena, Germany
Synopsis
When performing exercise induced 31P MRS motion and activities from the everyday life and the positioning of the investigated extremities within the MR scanner should be considered, since this could induce partial ischemia effects like muscular cramps. The aim of this study was to prove the effect of muscle tension on metabolic parameter measured by 31P MRS after an intense exercise. PCr recovery as well as pH kinetic was significantly slowed by a partial ischemia preventing a quantitative evaluation.
Purpose
Phosphorous MR spectroscopy (31P MRS) is a powerful tool to quantify metabolites in skeletal muscle in vivo. It provides information about cellular pH homeostasis dynamics by analyzing kinetics of pH, phosphocreatine (PCr) and inorganic phosphate (Pi) during exercise and recovery1. Motion and activities from everyday life as well as the positioning of the investigated extremities should, however, be carefully considered, since this could induce partial ischemic effects, like muscular cramps, due to a higher fiber tension. As a result, assessment of PCr recovery time constants or pH values may be corrupted and may complicate interpretations of metabolic conditions. The aim of this study was to prove the effect of two different pedal positions of an MR compatible ergometer on the kinetics of high-energy metabolites and acidification after intense exercise of the calf muscle.Methods
Two healthy volunteers (age: 23 and 22 years) participated in the study and performed series of unilateral plantar flexion by pushing the right foot against the pedal (3 min, 0.6 bar counter pressure, 100 bpm frequency)2. Both subjects performed two identical runs of this exercise. In the first run the pedal was unlocked during recovery which enables the ankle to stretch in a plantar flexion up to 125° relative to the leg. In the second run the pedal was locked resulting in a neutral position of the ankle and 90° relative to the leg (Figure 1). 31P MR spectroscopic (2D FID CSI, TR = 290 ms) data were acquired during the complete exercise (rest – exercise – recovery).Results
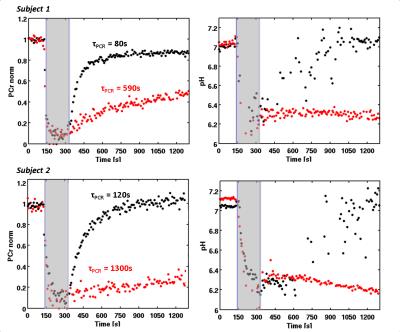
In terms of applied force both runs were comparable for subject 1 and 2, respectively (mean force for subject 1: 239 N and 221 N; subject 2: 197 N and 200 N). PCr decreased within 60 s below 20% of its initial level. Releasing the pedal after the exercise (125° relative to the leg) was associated with typical PCr (τPCr = 80 s and τPCr = 120 s for subject 1 and 2, respectively) as well as pH kinetics showing a short decrease immediately after the exercise due to the PCr synthesis followed by a continuous increase back to the resting pH value. In contrast, the fixated pedal (90° relative to the leg) led to calf muscle cramps as reported by the participants after the examination. This was also reflected by a significantly slower PCr recovery (τPCr = 590 s and τPCr = 1300 s) and constant low pH values during the post-load phase (see red dots in Figure 2). This pedal position also inhibited the removal of H+ and thus the acidification was stable for the whole duration of the recovery.Discussion and Conclusion
These two extreme cases demonstrate rather significant effects of muscle fiber tension on tissue perfusion and metabolic processes during recovery after muscular load. As a result this may lead to restricted blood flow and thus, an inhibited perfusion by an almost closed system. Since the main H+ washout is determined by the efflux, pH is not able to recover back to its resting value. Furthermore, the lack of oxidative ATP synthesis is significantly slowing down the PCr recovery and is additionally negatively affected by a stable low pH value in terms of suppressed creatine kinase3. Intense exercises may increase the tension on the fibers due to the connection between myofilaments4. With a heterogeneous flexibility of the lower extremities the amount of tension and thus the extent of the cramp on the calf muscle, is different among subjects. This hampers the metabolical evaluation or even quantitation of this partial ischemia and should be avoided in all circumstances.Acknowledgements
This work was supported by the Competence Centre for Interdisciplinary Prevention (KIP) atthe Friedrich Schiller University Jena and the German Professional Association for Statutory AccidentInsurance and Prevention in the Foodstuffs Industry and the Catering Trade (BGN). K.M.is supported by a graduate scholarship of the Friedrich-Schiller-University Jena (Landesgraduiertenstipendium).K.M. also acknowledges support by the German Academic ExchangeService (DAAD) for a short-term international scholarship at the University of Liverpool(57044996). The authors declare to have no relevant financial interests to disclose with regard tothis study.References
1. Kemp G. Muscle Studies by 31P MRS. eMagRes. 2015; 4(1): 525-34.
2. Tschiesche K et al. MR-Compatible Pedal Ergometer for Reproducible Exercising of the Human Calf Muscle. Med Eng Phys. 2014; 36(7): 933-37.
3. Schmid I et al. Exercising calf muscle T2* changes correlate with pH, PCr recovery and maximum oxidative phosphorylation. NMR Biomed. 2014; 27(5): 553-60.
4. Fitts R J The cross-bridge cycle and skeletal muscle fatigue. Appl Physiol. 2008; 104(2): 551-8.
Figures