1565
Semi-automated analysis of diaphragmatic motion with cine MRI in controls and non-ambulant Duchenne Muscular Dystrophy (DMD) patients1Imanova, London, United Kingdom, 2UCL, Institute of Child Health, London, United Kingdom, 3University College London, 4Imperial College London, United Kingdom, 5GlaxoSmithKline, Brentford, United Kingdom
Synopsis
This study presents both an analysis pipeline for measuring diaphragmatic motion from cine MRI data, and the application of this image processing technique to investigate exploratory MRI endpoints of respiratory function in both healthy controls and non-ambulant DMD boys. Cine-derived metrics of diaphragm motility and contractility correlated with sitting spirometry-derived forced vital capacity, and showed relationships with disease progression surrogates of age and months non-ambulatory, as well as a longitudinal change over 12 months. Longitudinal changes were not seen in spirometry measures.
Introduction
DMD
involves progressive muscle damage including of the diaphragm, ultimately
requiring ventilation1. Recent studies have described the spirometry
derived forced vital capacity (FVC) as a percentage of predicted normal values (%Pred)
in both ambulant and non-ambulant DMD2–4. Our aim was to explore whether image analysis of dynamic (cine)
MRI can be used to provide an adjunctive measure of diaphragm mobility in DMD.
Patients and Methods
Thirteen non-ambulant DMD boys
(mean age 13.2±2.1 years, 20.9±12.6
months since loss of ambulation) and 10 age-
and gender-matched healthy volunteers (mean age 14.6±1.3 years; P=0.081) were recruited and scanned at baseline, with a
subset (n=10) of the DMD subjects also assessed at follow-up visits 3, 6 and 12
months later.
Spirometry derived FVC,
%Pred, peak expiratory flow (PEF), maximal inspiratory pressure (MIP), and
maximal expiratory pressure (MEP) were recorded. 60 Cine MRI frames were acquired each
0.5s in sagittal acquisitions at the midline of each lung on a Siemens Skyra 3T
using a TR of 250ms, TE of 1.39s, FA 8°, and 5mm slice thickness.
A schematic of the image analysis pipeline, including the
lung and diaphragm measures extracted5,7, is provided in Figure 1.
Results
Strong differences in almost all lung shape parameters were
found between controls and DMD, controlling for height differences between the
groups (Table 1). There was good
correlation of most diaphragm motion measures with the spirometry measures of sitting
FVC (Table 2). The end-expiratory
lengths were shorter and a larger change in TDM is required in DMD patients for
a unit change in CSA (Figure 2). The end-expiratory length for ANT and PST were
numerically reduced at all follow-up visits, but only reached significance at 3
month follow-up (P=0.02 and 0.03, two-tailed).
Age and to a greater degree months of non-ambulation
influence the observed longitudinal decrease in min CSA, min TDM and max TDM
(Figure 7: left and middle plots). This is further supported by the
statistically significant relationships found for the number of non-ambulatory
months versus max CSA (R=-0.460, P=0.0042, N=37), delta CSA (R=-0.482, P=0.0025)
and mean CSA (R=-0.368, P=0.025), both max TDM (R=-0.394, P=0.016) and delta TDM
(R=-0.401, P=0.014), max DIA (R=-0.441, P=0.0062), delta DIA (R=-0.527, P=0.0008)
and mean DIA (R=-0.391, P=0.017), delta CNT (R=-0.438, P=0.0066), max PST (R=-0.338,
P=0.041) and delta PST (R=-0.358, P=0.030). In contrast, only the spirometry
metric of %Pred correlated with the months of non-ambulation (R=-0.390, P=0.0299
(N=31)). There was a statistically
significant decrease in min CSA at 12 months compared to baseline (-15.3%
(19.3), P=0.03, Figure 3 top-right). No longitudinal
changes were observed for FVC sitting or %Pred.
Discussion
Although spirometry is currently the most
common test for pulmonary function in DMD as an outcome measure in clinical trials it
requires a large number of subjects to account for the wide variability.
Furthermore, measures can be heavily reliant on subject cooperation and
motivation to perform the tests.
On
the other hand, MRI appears to offer a sensitive and targeted measure of
diaphragm function. While still requiring
cooperation, the manoeuvres required for the MRI assessment are less dependent
on coordination and following specific commands, and have been shown to be
similar in prone and supine positions9. The MRI parameters may
also offer a more extensive measurement and more flexibility to probe specific,
individual contributions to pulmonary function (such as the lower contribution from the anterior part of the diaphragm to the CSA change) since multiple imaging
planes and targeted regions-of-interest, on a slice-by-slice or volumetric
basis, can be objectively explored with relative ease and accuracy.
Previous studies exploring MRI measures of pulmonary function
have largely focused on healthy volunteers [5–9]. Cluzel et al. showed good
agreement between spirometry and MR values of lung volume. We have also demonstrated
that many of the derived MRI summary
measures correlate well with spirometry data but also other measures of disease
progression/severity (such as months of non-ambulation) in DMD.
In this DMD cohort, the finding of a statistically
significant decrease in min CSA at 12 months compared to baseline and a
trending decrease in min TDM across visits, could possibly be due to a
weakening of both the diaphragm and the intercostal muscles over the one year
study period. In addition, the trending decrease in max TDM across visits could
indicate progressive diaphragm dysfunction in this DMD cohort.
Acknowledgements
The financial support of L’Association Française contre les Myopathies (AFM) and European Commission (EU) are also acknowledged (VR). This study was supported by the National Institute for Health Research Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London (FM). The MRC support to the Neuromuscular Translational Centre at UCL, and the support of the Muscular Dystrophy Campaign to the Neuromuscular Centres at GOSH and UCLH is also gratefully acknowledged.
References
1. Bushby K, Finkel R, Birnkrant DJ, Case
LE, Clemens PR, Cripe L, et al. Diagnosis and management of Duchenne muscular
dystrophy , part 1?: diagnosis , and pharmacological and psychosocial
management. Lancet Neurol. Elsevier Ltd; 2010;9(1):77–93.
2. Ricotti V, Ridout DA, Scott E,
Quinlivan R, Robb SA, Manzur AY, et al. Long-term benefits and adverse effects
of intermittent versus daily glucocorticoids in boys with Duchenne muscular
dystrophy. J Neurol Neurosurg Psychiatry. 2013;84(6):698–705.
3. McDonald CM, Henricson EK, Abresch RT,
Florence JM, Eagle M, Gappmaier E, et al. The 6-minute walk test and other
endpoints in Duchenne muscular dystrophy: longitudinal natural history
observations over 48 weeks from a multicenter study. Muscle Nerve. 2013 Sep;48(3):343–56.
4. Buyse GM, Goemans N, Van Den Hauwe M,
Meier T. Effects of glucocorticoids and idebenone on respiratory function in
patients with duchenne muscular dystrophy. Pediatr Pulmonol. 2013;48(9):912–20.
5. Cluzel P, Similowski T, Chartrand-Lefebvre
C, Zelter M, Derenne JP, Grenier P a. Diaphragm and chest wall: assessment of
the inspiratory pump with MR imaging-preliminary observations. Radiology.
2000;215(2):574–83.
6. Gierada S, Strandt A, Prost W, Goodman
R. Diaphragmatic Motion?: in Healthy Subjects ’ Fast. Radiology. 1995;194:879–84.
7. Kondo T, Kobayashi I, Taguchi Y, Ohta
Y, Yanagimachi N. A dynamic analysis of chest wall motions with MRI in healthy
young subjects. Respirology. 2000;5(1):19–25.
8. Takazakura R, Takahashi M, Nitta N,
Murata K. Diaphragmatic Motion in the Sitting and Supine Positions: Healthy
Subject Study Using a Vertically Open Magnetic Resonance System. J Magn Reson
Imaging. 2004;19(5):605–9.
9. Tomita K, Sakai Y, Monma M, Ohse H, Imura S. Analysis of Diaphragmatic Motion with Prone Positioning Using Dynamic MRI. J Phys Ther Sci. 2004;16(2):85–9.
Figures
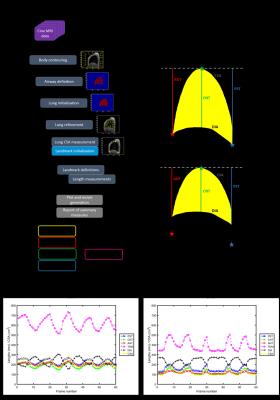
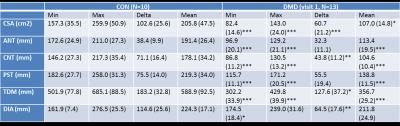
Table 1. Group mean(SD) of the summary measures at
baseline: cross-sectional area (CSA), anterior (ANT), central (CNT) and
posterior (PST) lung lengths, total distance of motion of the diaphragm (TDM),
and diaphragm length (DIA). *** P<=0.001, ** P<=0.01, * P<=0.05
two-tailed, DMD versus CON, height as covariate.
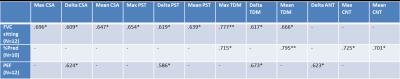
Table 2. Spirometry measures at baseline correlations
with MR-derived metric of diaphragm motion: cross-sectional area (CSA),
anterior (ANT), central (CNT) and posterior (PST) lung lengths, and total
distance of motion of the diaphragm (TDM). ** P<=0.01, * P<=0.05
two-tailed.
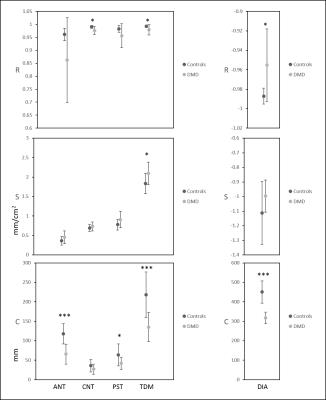
Figure 2. Mean
and SD of the correlation coefficient (R: top row), the slope (S: middle row)
and the y-axis intercept (C: bottom row) of the linear regressions for lung CSA
versus each of the length measures ANT, CNT, PST, TDM and DIA at baseline.
P-values for the two-sample t-tests, comparing controls to DMD, are indicated
as *** P<=0.001, ** P<=0.01, * P<=0.05.
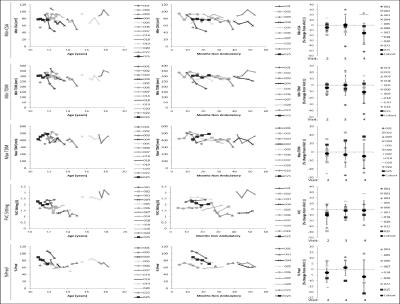
Figure 3. Diaphragm motion and spirometry measures age
(left), months non-ambulatory (middle), and longitudinal follow-up visit (right
plots). A decrease in lung and
diaphragmatic motion are notable as age and month non-ambulatory (surrogates of
disease progression) increase. Only the
maximum CSA (the deepest inspiration) showed a longitudinal effect, *
P<=0.05. Neither spirometry measure
showed a statistically significant change.