1562
Spiral Fingerprinting of Articular Knee Cartilage and Bone1Radiology, University of Cambridge, Cambridge, United Kingdom, 2IMAGO7 Research Center and IRCCS Stella Maris, Pisa, Italy, 3Radiology, University of Cambridge, 4GE Global Research, Munich, Germany
Synopsis
Magnetic resonance fingerprinting (MRF) seeks to acquire MRI parameter maps at shorter overall scan duration than achievable through separate image acquisitions. This work demonstrates multi-slice MRF at 3T for T1, T2, and proton-density mapping of a healthy human knee at a high in-plane spatial resolution (0.7x0.7mm$$$^2$$$) with an eight-channel transmit/receive coil at 3T. The spiral trajectories enabled quantification of tissues with ultrashort echo-times, such as trabecular bone.
INTRODUCTION
Musculoskeletal (MSK) disease is a leading cause of mobility impairment in the elderly worldwide (1). Early detection of MSK disease may improve therapies for disease treatment and prevention. MRI is a preferred tool in assessing MSK disease, such as for septic arthritis, osteomyelitis, and soft-tissue masses (2). MRI relaxation mapping is particularly promising for assessing osteoarthritis, where there is no clinically accepted method for its early detection (3).
Reducing the acquisition time of quantitative MRI (qMRI) methods can increase their use for disease detection. Quantitative T1, T2, and proton-density scans traditionally require separate sequences, leading to long overall scan durations. Patients with MSK disease may have difficulties remaining comfortable for the extended time periods required for qMRI, which also increases imaging costs and reduces qMRI utilisation.
Magnetic resonance fingerprinting (MRF) reduces scan duration by measuring multiple contrasts (such as T1, T2 and proton-density) in a single scan using pseudo-random scan parameters for encoding contrasts and reconstructing those via pattern matching to parameter dictionaries (4). Acquisitions that acquire data at ultra-short times are normally based on spiral or radial k-space trajectories and start sampling in the centre of k-space. This enables measuring signal with short T2* times, such as bone and cartilage. MRF techniques minimize their repetition time to increase steady-state free precession (SSFP) based contrast, which subsequently requires an increased number of spiral shots to achieve high resolution.
METHODS
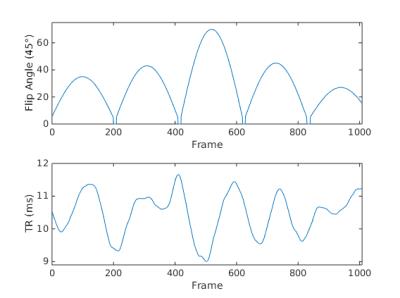
Axial images of a healthy human knee were obtained with ethical approval using 2D SSFP MRF (nominal voxel size = 0.70x0.70mm$$$^2$$$, field-of-view (FOV) = 18cm, matrix-size = 257x257, with twelve 3.5mm thick interleaved slices). MRF was measured on a 3T system (MR750, GE Healthcare, Milwaukee, WI, USA) with a 250kHz bandwidth, 144 golden-angle interleaves, 1008 frames with 1024 points acquired per frame, and equipped with an 8-channel transmit/receive knee coil. The total scan time was 2min39sec. The pseudorandom TRs and flip angles are shown in Figure 1. The images were reconstructed following a dictionary pattern matching technique (4) containing T1 values from 20ms to 2.5s and T2 values from 5 to 200ms.RESULTS
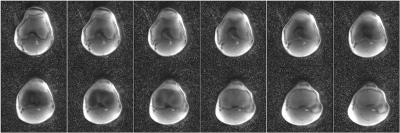
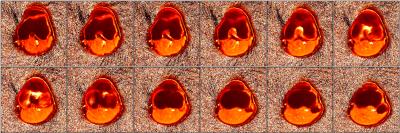
The reconstructed MRF maps are shown in Figure 2. The T1 and T2 measurements of femoral trabecular bone are (T1 = 117±13ms, T2 = 22±3ms) and articular cartilage (T1 = 550±220ms, T2 = 66±17ms). There are minor hyperintense regions laterally on the proton-density image due to coil inhomogeneities. Despite this hyper-intensity, the T1 and T2 maps do not display any hyperintense effects in these regions. The reconstruction time for all twelve slices was 10 minutes.DISCUSSION
This work demonstrates 12-slice, 8-channel MRF of a healthy human knee at a high in-plane spatial resolution (0.7x0.7mm$$$^2$$$, nominal) for T1 and T2 mapping of a healthy human knee. Radial fingerprinting has previously been demonstrated within the hip, achieving a nominal 0.8x0.8mm$$$^2$$$ resolution without local receive coils (5). The T2 values of the knee (~66ms) were higher than the expected healthy values obtained with fast-spin echo methods (30-45ms) (6), while the T1 values were lower (~550ms with MRF vs ~1200ms) (7). The spiral trajectory of MRF enabled bone mapping. This scan time (2min39sec) is short when compared to methods that require the same imaging time per map, thus tripling the time (8,9). The number of slices could be increased and thickness reduced for higher resolution imaging without sequence modification, although slice number increases will result in a proportional increase in imaging and reconstruction time. Image reconstruction time can be reduced through code optimizations. B1 pattern matching will improve the images with transmit-receive inhomogeneity correction. B0 and gradient delay correction will further improve the voxel point-spread-function.CONCLUSION
This work demonstrates MRF of a healthy human knee at a high in-plane spatial resolution (0.70x0.70mm$$$2$$$) with 8-channel transmit/receive coil for T1, T2, and proton-density mapping. The scan time (2min39sec) required to obtain 12 slices of these parameter maps was shorter than required by traditional methods.Acknowledgements
This work was supported by the Royal Society, GE Healthcare and GlaxoSmithKline.References
1. Chen A, Gupte C, Akhtar K, Smith P, Cobb J. The global economic cost of osteoarthritis: how the UK compares. Arthritis 2012;2012.
2. Karchevsky M, Schweitzer ME, Morrison WB, Parellada JA. MRI findings of septic arthritis and associated osteomyelitis in adults. American Journal of Roentgenology 2004;182(1):119-122.
3. Mosher TJ, Dardzinski BJ, Smith MB. Human Articular Cartilage: Influence of Aging and Early Symptomatic Degeneration on the Spatial Variation of T2—Preliminary Findings at 3 T 1. Radiology 2000;214(1):259-266.
4. Ma D, Gulani V, Seiberlich N, Liu K, Sunshine JL, Duerk JL, Griswold MA. Magnetic resonance fingerprinting. Nature 2013;495(7440):187-192.
5. Cloos MA, Alon L, Geppert C, Sodickson DK, Lattanzi R. Rapid T1 and T2 mapping of the hip articular cartilage with radial MR fingerprinting. Proc Intl Soc Mag Reson Med 2015;23:0113.
6. Smith HE, Mosher TJ, Dardzinski BJ, Collins BG, Collins CM, Yang QX, Schmithorst VJ, Smith MB. Spatial variation in cartilage T2 of the knee. Journal of Magnetic Resonance Imaging 2001;14(1):50-55.
7. Gold GE, Han E, Stainsby J, Wright G, Brittain J, Beaulieu C. Musculoskeletal MRI at 3.0 T: relaxation times and image contrast. American Journal of Roentgenology 2004;183(2):343-351.
8. Stahl R, Blumenkrantz G, Carballido-Gamio J, Zhao S, Munoz T, Le Graverand-Gastineau MH, Li X, Majumdar S, Link T. MRI-derived T2 relaxation times and cartilage morphometry of the tibio-femoral joint in subjects with and without osteoarthritis during a 1-year follow-up. Osteoarthritis and Cartilage 2007;15(11):1225-1234.
9. Mamisch TC, Dudda M, Hughes T, Burstein D, Kim YJ. Comparison of delayed gadolinium enhanced MRI of cartilage (dGEMRIC) using inversion recovery and fast T1 mapping sequences. Magnetic Resonance in Medicine 2008;60(4):768-773.
Figures