1548
T2 -mapping and dGEMRIC of the patellar cartilage - long term follow-up after patellar stabilizing surgery in childhood1Department of Women´s and Children´s Health, Karolinska Institutet, Stockholm, Sweden, 2Department of Diagnostic Radiology, Oulu University Hospital, Finland, 3Department of Paediatric Radiology, Karolinska University Hospital, Sweden, 4Department of Molecular Medicine and Surgery, Karolinska Institutet, Sweden, 5Stockholm Sports Trauma Research Center, Karolinska Institutet, Sweden, 6Department of Orthopedics, Lund University, Sweden
Synopsis
Recurrent patellar dislocation in childhood often require surgical stabilization, but the effects on cartilage quality after surgery is unknown. 17 patients were examined with T2 and dGEMRIC ≥5 years after surgery. dGEMRIC was shorter centrally, whereas T2 was longer most medially in the patellar cartilage of the operated patella (p<0.05). The short dGEMRIC indicates loss of glycosaminoglycans in the patella of the operated knee. Longer T2 may be an early sign of joint pathology. These findings may indicate an imbalance in the synthesis of matrix molecules, a sign of early cartilage degeneration.
Purpose:
The identification of early degenerative cartilage changes remains a diagnostic challenge. The purpose of this study was to examine the cartilage quality in very young adults operated with a patellar stabilizing procedure in childhood, using both T2 mapping and post contrast T1 (dGEMRIC). In addition, we wanted to study whether cartilage quality, assessed with MRI, correlated to clinical parameters such as patellar instability and patient reported outcome (PRO).Methods:
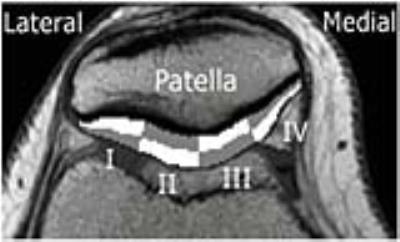
17 young adults with patellar instability and subsequent patellar stabilizing surgery in childhood were investigated ≥5 years (mean 11.6) after the operation. The study was approved by the Human Ethics Committee, Karolinska Institutet, Sweden. PRO was assessed by KOOS1,2. MRI examination of both the operated and the asymptomatic knee was conducted in a Philips Achieva®1.5T scanner with a dedicated knee coil. T1 and T2 analyses were performed in a centrally positioned axial slice of the patellar cartilage. T2 mapping was performed pre-contrast, and quantitative T1 analysis (dGEMRIC) 2 hours after an i.v. injection of 0.2 m M/kg Gd-DTPA2. The dGEMRIC values were corrected for BMI differences3.The macroscopic appearance was evaluated according to the modified Outerbridge classification of cartilage 4. A region of interest (ROI) was drawn to cover all patellar cartilage. Using the MOKKULA software (eveliina.lammentausta@oulu.fi), this ROI was divided into 4 deep and 4 superficial cartilage sectors (figure 1). SPSS® was used for the statistical evaluation and the level of significance was set at p ≤ 0.05. Paired T-test was used to compare MR parameters between operated and reference knees. When analyzing PRO, non-parametric tests were used.
Results:
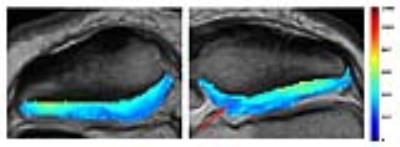
Comparing the operated to the heathy control side, the T2 bulk values of the operated knee was significantly longer in the most medial sector (IV) (p=0.023). When the cartilage was analyzed in a deep and a superficial half, the difference was located in the deep half of sector IV (p= 0.016) (figure 2). The dGEMRIC bulk value was significantly shorter in the central sector of the medial patellar cartilage III (p=0.048) of the operated side. Further evaluation revealed that in sector III, a significant difference was seen in the deep cartilage (p= 0.016) (figure 3). Neither T2 nor dGEMRIC differed between the operated and the reference knee regarding the superficial half of the cartilage. The cartilage changes could not be explained by macroscopic lesions, or reduced cartilage height (figure 4). The patients scored lower than age-matched controls2 in all of the five subscales of the KOOS. Degenerative changes detected by dGEMRIC was correlated to lower scores in the KOOS subscales Symptom, and Sports & Recreation (p=0.041, r=0.50), but not to sex, age at injury, recurrence rate, activity level or follow-up time.Discussion:
To our best knowledge, this is the first study using both T2 mapping and dGEMRIC to evaluate patellar cartilage in patients operated due to recurrent patellar dislocation in childhood. Despite young age and regained patellar stability, both T2 and dGEMRIC index differed between operated and control knees. Longer T2 is associated with cartilage degeneration as a result of cartilage swelling secondary to disruption of the collagen network, but may also be influenced by glycosaminoglycan concentration and macromolecular interactions5. Notably, the decreased dGEMRIC index was found only in the deep half, and not in the superficial half of the cartilage. For comparison, a previous study of patients with non-operatively treated recurrent patellar dislocations detected low dGEMRIC index in the superficial half of the cartilage6. Several factors may contribute to the depth wise differences between the studies; most likely both the initial instability and the operation contribute to early cartilage degeneration, perhaps by different mechanisms. There may be a shift in load from lateral to medial (corresponding to our sector III) after patellar stabilizing surgery7–9. It could be hypothesized that increased focal strain in sector III after surgery has impeded the synthesis of proteoglycans in the deep cartilage layer10,11, but further research in this field is needed.Conclusion:
The short dGEMRIC values centrally indicate loss of glycosaminoglycans in the patella of the operated knee. The increase in T2 may also indicate early stage cartilage pathology, possibly related to disruption of the collagen network. In addition, we found a correlation between dGEMRIC index and clinical symptoms, indicating better cartilage quality in patients with less pain and higher physical activity.Acknowledgements
The study was supported by H.R.H. King Oscar II´s and H.R.H. Queen Sophia´s Golden Wedding foundation, Capio Research foundation, Sophiahemmet Research foundation and Swedish National Centre for Research in Sports, Skandia Research Foundation, Promobilia Research foundation and Trygg-Hansa Research Foundation.
References
1. Roos EM, Roos HP, Lohmander LS, Ekdahl C, et al. Knee Injury and Osteoarthritis Outcome Score (KOOS)--development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88-96.
2. Paradowski PT, Bergman S, Sundén-Lundius A, et al. Knee complaints vary with age and gender in the adult population. Population-based reference data for the Knee injury and Osteoarthritis Outcome Score (KOOS). BMC Musculoskelet Disord. 2006;7(1):38.
3. Tiderius C, Hori M, Williams a, et al. dGEMRIC as a function of BMI. Osteoarthritis Cartilage. 2006;14(11):1091-1097.
4. Outerbridge RE. The etiology of chondromalacia patellae. 1961. Clin Orthop Relat Res. 2001;(389):5-8.
5. Burstein D, Bashir A, Gray ML. MRI techniques in early stages of cartilage disease. Invest Radiol. 2000;35(10):622-638.
6. Bengtsson Moström E, Lammentausta E, Finnbogason T, et al. Pre- and postcontrast T1 and T2 mapping of patellar cartilage in young adults with recurrent patellar dislocation. Magn Reson Med. 2015;74(5):1363-1369.
7. Mani S, Kirkpatrick MS, Saranathan A, et al. Tibial Tuberosity Osteotomy for Patellofemoral Realignment Alters Tibiofemoral Kinematics. Am J Sports Med. 2011;39(5):1024-1031.
8. Kuroda R, Kambic H, Valdevit A, et al. Articular cartilage contact pressure after tibial tuberosity transfer. A cadaveric study. Am J Sports Med. 2001;29(4):403-409.
9. Sambasivarao S V, Saranathan A, Kirkpatrick MS, et al. The Effect of Tibial Tuberosity Realignment Procedures on the Patellofemoral Pressure Distribution. Knee Surg Sport Traumatol Arthrosc. 2012;20(10):2050-2057.
10. Buschmann MD, Hunziker EB, Kim YJ, et al. Altered aggrecan synthesis correlates with cell and nucleus structure in statically compressed cartilage. J Cell Sci. 1996;109:499-508.
11. Grodzinsky A,
Levenston ME, Jin M, et al. Cartilage tissue remodeling in response to
mechanical forces. Annu Rev Biomed Eng. 2000;2:691-713.
Figures