1546
T1ρ assessment of cartilage in a large animal model of traumatic joint injury1Radiology, University of Iowa, Iowa City, IA, United States, 2Orthopedics & Rehabilitation, University of Iowa, Iowa City, IA, United States
Synopsis
MRI offers the opportunity for early characterization of cartilage in injured joints with emerging contrast mechanisms such as T1ρ. The purpose of this work was to investigate the ability of T1ρ to detect early cartilage changes in a large animal (goat) model of PTOA. T1ρ imaging of injured joints showed increased relaxation times six months after injury compared to uninjured contralateral joints in five animals. T1ρ may help better elucidate the time course and mechanisms of cartilage degeneration in PTOA.
Introduction
Osteoarthritis (OA) is the second leading cause of morbidity and disability in the United States and results in a high economic burden. An important subset of patients develop OA from traumatic joint injury that initiates the disease process. Post-traumatic OA (PTOA) tends to afflict younger and more physically active subjects, amplifying the physical and economic effects as these patients may become debilitated during their working years of life and are more likely to require joint replacement at a relatively young age.
While radiographic evidence remains the clinical gold standard for OA diagnoses, such evidence of the degradation and loss of cartilage associated with OA may appear many years after the cascade of irreversible degenerative processes has begun. The resolution and outstanding soft tissue contrast of MRI offers the opportunity for early characterization of cartilage in injured joints.1 Emerging contrast mechanisms such as T1ρ relaxation have the potential to detect early stages of OA,2 when interventions may be possible to slow or forestall these degenerative processes. The purpose of this work was to investigate the ability of T1ρ to detect early cartilage changes in a large animal model of PTOA.
Methods
Following an IACUC-approved protocol, five skeletally mature, castrated male goats were anesthetized and underwent an operation to introduce traumatic joint injury to the left knee. The procedure consisted of a partial meniscectomy of the anterior horn of the medial meniscus followed by a blunt impact to the weight bearing region of the medial femoral condyle using a hand-held impaction device.3 The original impact was approximately 6 mm in diameter and the trauma energy was 1.2 Joules. Following surgery, the goats roamed freely at pasture for a period of 24 weeks. At this time point, animals were euthanized and the impacted left knee and corresponding uninjured right knee were imaged intact. Scans were performed on a 3T Siemens Tim Trio scanner (Siemens, Erlangen, Germany).
T1ρ weighted images derived from a segmented 3D GRE-based pulse sequence4 with a conventional T1ρ preparation block (+90° tip-down, spin lock durations of 10, 20, 40, and 60ms at 400Hz amplitude, -90° tip-up and crusher gradient, see Figure 1) applied prior to acquisition of segments of 16 centrically ordered k-space lines. Additional imaging parameters were intrasegment TR/TE = 9.4/4.7ms, intersegment repetition time = 1000ms, FOV = 16cm x 16cm with 256x128 matrix covering 20 slices, BW=260 Hz/pixel for a total scan time of 2:10 per spin lock time (8:40 per complete set). Images were acquired in the sagittal plane, selected to correspond to a 2D T2-weighted fat saturated acquisition for correlated morphology. T1ρ relaxation times were calculated using a monoexponential nonlinear least squares fit. Mean T1ρ relaxation times were computed for contours defined at consistent locations along the lateral femoral condyle for both left (injured) and right (control) knees and compared for each animal.
Results
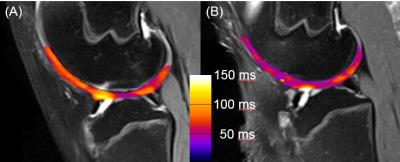
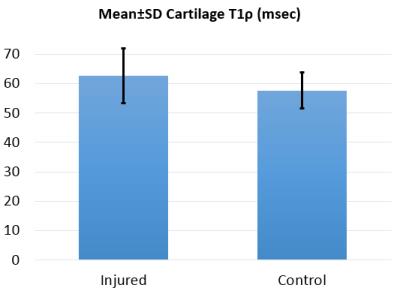
Figure 2 shows sample pairs of injured and control knees with cartilage T1ρ relaxation time overlaid onto T2-weighted morphologic images, where the increased T1ρ is evident in the injured limb suggestive of degenerative cartilage changes. Mean T1ρ times trended consistently higher in the injured knee compared to the control knee in all five specimens, with injured knees having increased T1ρ by an average of 9% over the five samples, though the the difference did not reach statistical significance in this small sample (Figure 3).Discussion
This work demonstrates the feasibility of generating T1ρ assessments of cartilage condition in a large animal model of PTOA. T1ρ continues to show promise as an imaging biomarker for early detection of cartilage changes in vivo, trending towards increased relaxation times in injured cartilage as compared to the contralateral control. Further application of T1ρ relaxometry in this model in larger and longitudinal studies may potentially better elucidate the time course and mechanisms of cartilage degeneration in PTOA.Acknowledgements
Funded by NIH/NIAMS AR055533.References
[1] Chu CR et al. Early diagnosis to enable early treatment of pre-osteoarthritis. Arthritis Res Ther 2012;14:212
[2] Choi JA, et al. MR imaging of articular cartilage physiology. Magn Reson Imaging Clin N Am. 2011;19:249.
[3] Heckelsmiller DJ et al. A Handheld Device for Creating Cartilage Blunt Impact Injuries. In: 62nd Annual Meeting of the Orthopaedic Research Society; 2016 Mar 3-5; Orlando, FL.
[4] Li X et al. In vivo T1ρ mapping in cartilage using 3D magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots (3D MAPSS). Magn Reson Med 2008;59:298.
Figures