1504
Simultaneous Multi-Volume 4D Phase Contrast Flow MRIDavid Feinberg1,2 and Liyong Chen1,2
1Advanced MRI Technologies, LLC, Berkeley, CA, United States, 2Helen Wills Neuroscience Institute, Univ of California, Berkeley, Berkeley, CA, United States
Synopsis
A new technique of simultaneous 4D flow imaging acquired with simultaneous multiple 3D volumes (SMV) is presented. The velocity measurements are compared to conventional 4D flow imaging and have very good correspondence.
Introduction
4D Phase contrast (PC) MRI is currently a popular approach to flow imaging1 in which quantitative velocity information is measured in a 3D volume of the head or body. In conventional PC imaging the bipolar gradient pulse is used for linear velocity encoding of signal phase2 and combined with velocity phase maps3. Our previous work developed and evaluated simultaneous multi-slice (SMS) in 2D PC flow imaging to increase the number of 2D PC images without increasing the scan time4. Here we develop simultaneous multiple 3D volume (SMV), multi-slab imaging, as a novel 4D PC flow imaging technique.Methods
A 3D FLASH GE sequence with cine acquisition and prospective cardiac gating was modified to excite with multiband (MB) rf pulsesand bipolar velocity gradient. Imaging was performed in 6 normal subjects with both single-3D volume and SMV 2 slab volumes or 3 slabs in head and thorax. Our 3T scanner (Siemens Trio) is located in a neuroscience center and had limited coils available, a spine coil could only be used for thorax imaging which reduced the spatial sensitivity and image quality in neck and chest imaging. A 32 channel coil was available for head imaging. Imaging parameters for cine 3D SMV and conventional 3D GRE PC are: TR=6.16ms, TE=3.92ms, FA Angle=15°, multiband rf pulse width=2.0ms, IPAT acceleration factor=2. For brain, the matrix size and FOV=190x154mm2, matrix=(128x64) slab thickness= 32mm, (16 x 2mm slice). For thorax imaging: FOV=340x234mm2, matrix=(128x88), slab thickness= 48mm, (16 x 3mm slice). Acquisitions evaluated 2 or 3 slabs (SMV=2 or 3) with a 100% slab spacing, as shown in Figure 1. The interleaving of two SMV acquisitions gave complete coverage with either 4 slabs or 6 slabs, respectively. Venc was either 80 cm/s for head or 160 cm/s for thorax.Results
Fig. 2 shows the spatial coverage in magnitude images in a single SMV-3 slab acquisition of PC head and neck imaging. The total scan time was 8-9 minutes, depending on heart rate, for SMV-2 or SMV-3 and conventional SMV=1 4D PC acquisitions. There was no loss in spatial resolution using SMV acquisitions. Figs3 and 4 shows representative magnitude and phase maps in the interleaved SMV-2 and SMV-3 acquisitions in the vessels with very good match between the velocity curves from conventional single slab 4D PC and SMV multi slab in head and thorax, Figs 5 and 7, respectively. Our scanner only having a spine and neck array (combined 12 channels) but no anterior chest coil array, the SNR and coil sensitivity limited the great vessel imaging. There was expected slab profile relate signal loss and aliasing in the outermost 2 slices of each slab. There is also signal reduction in the lower slab in the neck as the 32 channel head coil had lower sensitivity in the lower head and neck regions. In these experiments we did not overlap the interleaved slabs to account for the outer slice losses, although this could easily have been achieved, Figs. 3-4. There was high correspondence in the measured velocity curves using multi-slab and conventional single slab acquisitions, shown in Figs 5 and 7.Discussion
While not evaluated here, the use of thinner 3D volumes with SMV will allow more consistent inflow of spins throughout the width of the slab, whereas a thicker 3D slab covering the same region could have reduced inflow depending on velocity and time of flight distance of blood flow. There was no limitation due to SAR or peak rf power in these studies due to the low flip angle and low MB factor of 2 or 3 used. The acceleration factors and acquisition speed were not fully optimized in these initial experiments, such as higher in-plane acceleration, compressed sensing and higher SMV factor. The implementation of controlled aliasing will allow higher accelerations and improved image quality. The implementation using proper coil arrays may allow closer spaced slabs and higher SNR. One potential disadvantage of the technique is the requirement of two acquisitions for contiguous volume coverage.Conclusion
A new technique of 4D flow imaging using simultaneously acquired multiple 3D volumes is presented and shows good correspondence in quantitative velocity measurement to conventional 4D flow imaging.Acknowledgements
NIH R44MH112210References
1. Markl et al JMRI 2012. 2. Hahn J Geophys 1960 3. Feinberg DA, Diagnostic Imaging,1984. 4. Feinberg DA, Chen L,page 320, ISMRM 2016.Figures
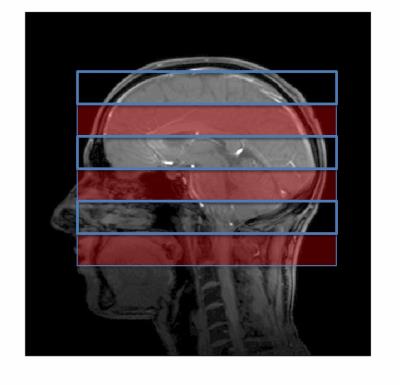
Figure 1. Scout view of simultaneous 3 slabs (solid
red) with 100% slab spacing for interleaved
second acquisition (blue line).
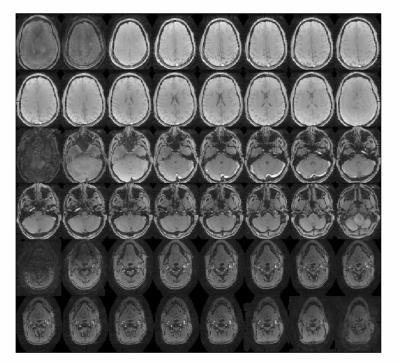
Figure 2. Magnitude images of 3 slab PC image acquisition of head and neck.
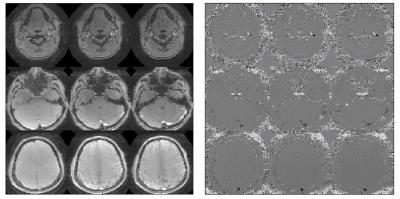
Figure 3. Average SMV-3 PC magnitude and
phase image averaged over time series.
(rows are subset of 3 corresponding slabs).
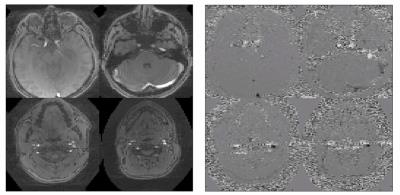
Figure 4. Average magnitude and
phase image from each slab of two
interleaved SMV-2 slab acquisitions for 4 slabs total.
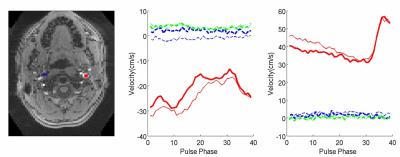
Figure 5. Comparison
of conventional 4D PC to SMV-2 4D PC imaging.
SMV (dark lines) and conventional single slab (thin line). Plot of
jugular vein (left) and ICA (right). The red, blue and green curves are
through-plane, AP, LR direction,
respectively.
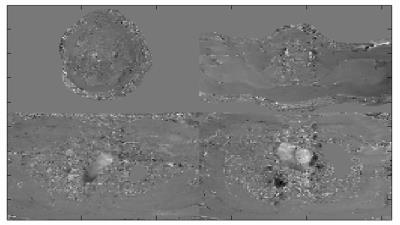
Figure 6. Average PC phase
image over time series of SMV-2
acquisition interleaved for 4 slabs total.
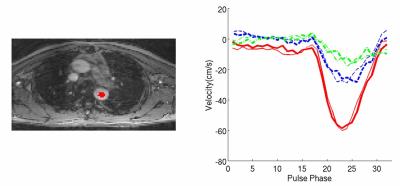
Figure 7. Velocity curve comparison of SMV-2 and conventional single slab 4D
PC measurements: SMV (dark curve) and conventional (light curve), with color
corresponding to different velocity directions, red, blue and green curves are
through-plane, AP, LR direction,
respectively.