1502
Dual Echo Trajectory : Comparison to Partial Fourier Acquisition and Sequence Optimization1Department of Electrical and Computer Engineering, Seoul National University, Seoul, Korea, Republic of
Synopsis
In this study, we compared two acceleration methods in spin echo imaging; previously proposed Dual Echo Trajectory (DuET) and partial Fourier acquisition. Also, we further improved DuET with interleaved multislice acquisition and echo timing correction.
Purpose
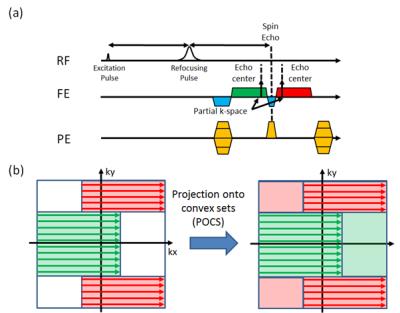
The significance of spin echo imaging comes from its robustness to field inhomogeneity artifacts. Conventional spin echo imaging acquires a single line of K-space every excitation. This strategy combined with a long relaxation time results in a long scan time. To accelerate the data acquisition, we have previously introduced the Dual Echo Trajectory (DuET).1 This new method acquires two partial echoes immediately before and after the spin echo time to accelerate data acquisition by two-fold and minimizes artifacts resulting from the phase difference between the two echoes. A brief summary of the method is summarized in Figure 1. In this work, we compared the partial Fourier acquisition, a widely used way of accelerating spin echo imaging, and DuET. In addition, the sequence was further optimized by adding two techniques; an interleaved multislice acquisition and echo timing correction similar to EPI.2Method
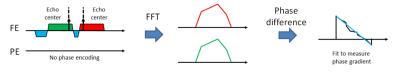
The interleaved multislice acquisition was implemented to DuET. For EPI-like echo timing correction, an additional DuET readout was acquired without phase encoding during a dummy TR, which was originally inserted for the signal to reach a steady-state. The readout data were compared to estimate readout imaging difference between the two echoes. The difference was compensated during reconstruction in K-space. The process is shown in Figure 2. To compare the proposed method and conventional spin echo imaging, a phantom and a healthy subject were scanned at a 3T scanner. For both scans, an off-resonance RF pulse was used to saturate fat signal to avoid artifacts in the proposed method. The scan parameters were as follows: 16 slices for in-vivo, 1 slice for phantom, FOV = 256×256 mm2, resolution = 1×1 mm2, slice thickness = 5 mm, TR/TE = 600/13 ms, gap time Δt = 0.78 ms, and center strip width = 128 samples, and acquisition time = 2.56 min for spin echo and 1.31 min for DuET. For the in-vivo scan, an additional spin echo image was acquired to generate partial Fourier acquisition images with different acquisition ratio ranging from 50 % to 75 %. Projection onto convex sets (POCS) algorithm was used to reconstruct partial Fourier acquisition.3 The reconstructed images will be compared to DuET in terms of normalized-root-mean-squared error (nRMSE) to original full K-space image calculated by the following equation.
$$nRMSE =\sqrt{\frac{\sum{(I_{DuET}-I_{SpinEcho})^2}}{\sum{I_{SpinEcho}^2}}}$$
Brain region was manually masked out when computing nRMSE. To show the effect of interleaved multislice technique and echo timing correction in DuET, additional single slice acquisitions were obtained.
Results
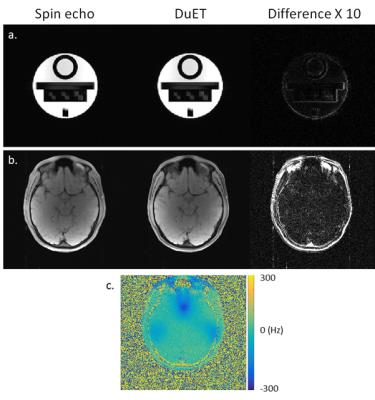
Figure 3 shows a comparison of conventional spin echo and DuET images. Phantom images (Figure 3-a) show that noticeable error occurs only on the edges. Such error is expected since DuET utilizes POCS. However, overall error is small (nRMSE = 1.33 %). The in-vivo images (Figure 3-b) show that difference is significant in non-brain regions, which have fast T2* decay. Other brain regions display only noise-like difference map (nRMSE = 4.28 %). It is also noticeable that the difference map is not dependent on local field inhomogeneity (Figure 3-c), which reaches 250 Hz in maximum. This implies that DuET does not change the image contrast nor introduce additional variance from field inhomogeneity.
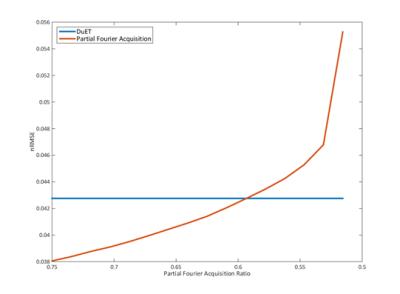
Figure 4 shows the nRMSE plot of partial Fourier acquisition and DuET. Simulated partial acquisition ranges from 50 % to 75 %. It is expected that 75 % partial Fourier acquisition performs better than DuET which also acquired 75 % of K-space in this work since the difference between two acquired echoes can be an additional error source. However, the crossing point of two nRMSE plots is calculated when acquisition ratio is 60 %. Since 60 % acquisition ratio takes 16 % more acquisition time than DuET, DuET has some advantages over partial Fourier acquisition.
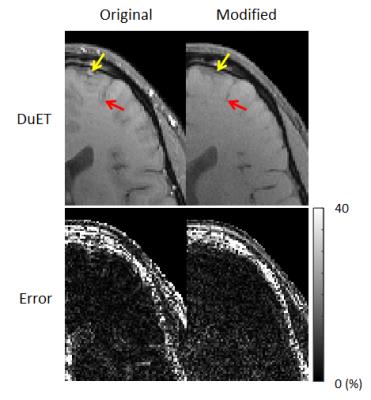
Figure 5 shows the comparison between the original DuET and DuET modified in this work. Significant error reduction is achieved near vessels, which are indicated by red arrows. Since interleaved multislice acquisition applies continuous excitation and dephasing on flowing blood, it results in suppression of vessel signals. Because vessels are the main source of reconstruction error in partial Fourier reconstructions such as POCS used in DuET, suppression of vessel signals led to the reduction in artifacts. Echo timing correction further reduced the artifact.
Conclusion
In this work, we further improved DuET by implementing interleaved multislice acquisition and echo time correction. The comparison between conventional spin echo imaging and DuET showed that it does not the image contrast and the image is still robust to field inhomogeneity artifact. The future work includes applying DuET to fast spin echo imaging.Acknowledgements
This research was supported by the Brain Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2015M3C7A1031969) and Brain Korea 21 Plus Project in 2016.References
1. J. Kim, J. Lee, Dual Echo Trajectory for Novel Fast Acquisition, ISMRM 2016
2. M. A. Bernstein, K. F. King, K J. Zhou, Handbook of MRI pulse sequences, Boston, MA; Academic press, 2004
3. EM Haacke, ED Lindskog, W Lin. A fast, iterative partial Fourier technique capable of local phase recovery. J Magn Reson 1991; 92:126–145.
Figures