1485
Full-FOV, whole-brain, half-millimetre in-plane readout-segmented EPIK for high-resolution fMRI studies1Institute of Neuroscience and Medicine, Medical Imaging Physics (INM-4), Forschungszentrum Juelich, Juelich, Germany, 2Faculty of Medicine, Department of Neurology, JARA, RWTH Aachen University, Aachen, Germany
Synopsis
Since the advent of EPI, numerous approaches have been suggested to enhance its resolution for high-resolution fMRI. Recently, several methods were demonstrated for fMRI with a sub-millimetre resolution. However, none of them can achieve such resolution with a full-FOV and, at the same time, with whole-brain coverage. This work aims to develop a novel imaging method based on EPIK in combination with readout-segmentation to achieve half-millimetre resolution with a full FOV. Here, under a typical fMRI constraint (TR of 3 s), the method was shown to provide 93 slices when further combined with the multi-band technique.
Purpose
Since the advent of EPI, numerous approaches have been suggested to enhance its resolution for high-resolution fMRI. Recently, several methods (e.g. PROPELLER-EPI1 or zoomed GRAPPA2) were demonstrated for fMRI with a sub-millimetre resolution. However, none of them can achieve such resolution with a full-FOV and, at the same time, with whole-brain coverage. For this purpose, this work proposes an imaging method using EPI with keyhole (EPIK)3,4 in combination with readout-segmentation5. EPIK has been previously demonstrated for high-resolution fMRI (1.0 mm2)6 and hence, we were motivated to utilize it as a base method here. The present work i) demonstrates the combination of EPIK with readout-segmentation and ii) verifies the capability of the method to yield half millimetre resolution with a full-FOV and whole-brain coverage.Methods
Figure 1a shows the schematic representation of the k-space trajectory for three-shot EPIK. Each measurement scans the central k-space region (keyhole) completely, whilst the peripheral k-space regions (sparse) are sparsely sampled with Δky' = 3/FOV. By sharing the sparse region data from three consecutive scans, the entire periphery of k-space can be completely constructed; crucially, a sliding window technique was used to ensure that the keyhole and the periphery of k-space are continually updated. Furthermore, this example features one-fourth of k-space as the keyhole region. Thus, the total number of phase encoding lines to be sampled reduces to 1/2 of that for comparable EPI. This scheme suggests efficient sampling along the phase encoding direction, however, the current keyhole region still contains peripheral information along the readout direction. Hence, for more efficient sampling on the central k-space, this work proposes to apply readout-segmentation to the keyhole region. As illustrated in Fig. 1b, readout-segmentation is combined such that the peripheral parts (marked by dotted-squares) are sampled once every two scans whilst the central k-space is still sampled at every scan; the horizontal size of the central keyhole part was set as one-fourth of the complete line. Similar to the EPIK reconstruction, the peripheral parts can be completed by sharing the data from two consecutive scans in a sliding window fashion. For method validation in comparison to original EPIK, the above configuration was employed in an in vivo measurement with the following imaging parameters: FOV = 240 × 240 mm2, matrix size = 160 × 160 (1.5 × 1.5 mm2), FA = 90°, TR/TE = 2000/60 ms, slice thickness = 3 mm and 12 slices. After validation, readout-segmented EPIK was exploited for half-millimetre resolution imaging with the following imaging condition: FOV = 210 × 210 mm2, matrix size = 416 × 416 (0.5 × 0.5 mm2), FA = 90°, TR/TE = 2000/35 ms, slice thickness = 3 mm, keyhole lines = 56, 4-fold acceleration and partial Fourier imaging (5/8). All experiments were performed on a Trio 3T MRI scanner (Siemens, Germany) with a 32-channel phased array coil.Results
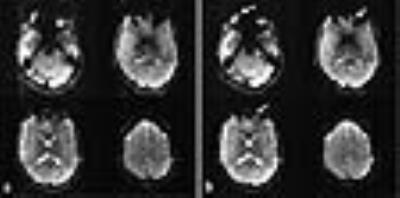
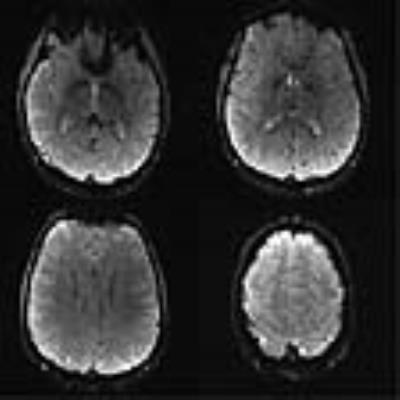
Figure 2 shows reconstructed EPIK (1.5 × 1.5 mm2) and readout-segmented EPIK (1.5 × 1.5 mm2) scans. Visual inspection of the figure suggests that they were well reconstructed without any severe artefacts. However, due to the faster readout in readout-segmented EPIK, its reconstructed images exhibit reduced geometric distortions than the EPIK images; additionally, reduced ghost artefacts were also observed for all slices. Figure 3 shows 4 representative slices from a high-resolution readout-segmented EPIK (0.5 × 0.5 mm2) dataset. The figure demonstrates that all slices were well reconstructed without any significant degradation arising from ghosts or image blurring. Although the image SNR is relatively low due to the half millimetre resolution and the relatively low field strength of 3T, a detailed spatial representation of the anatomical structures such as gyri or sulci is observed in all slices.Discussion and Conclusions
The combination of readout segmentation with EPIK was demonstrated at 3T and its performance was evaluated in comparison with original EPIK. Its use was exploited for half-millimetre resolution imaging and the images were successfully reconstructed. Here, the minimum TR for a single slice was 96.72 ms, implying that the method can achieve 31 slices when TR is given as 3 s (a typical TR employed for fMRI). Furthermore, if the multi-band acceleration (e.g. R = 3) is additionally integrated,7 brain coverage with 93 slices is also possible. Here, high-resolution imaging was demonstrated with a TE of 35 ms. However, the TE can be further decreased by a further optimization of the size of keyhole and skipping part of k-space by employing the partial Fourier technique. This suggests the use of method for columnar resolution fMRI at ultra high fields.Acknowledgements
No acknowledgement found.References
1. Krämer M, Reichenbach JR. High resolution T2*-weighted magnetic resonance imaging at 3 Tesla using PROPELLER-EPI. Z Med Phys. 2014;24:164–173.
2. Heidemann RM, Ivanov D, Trampel R, et al. Isotropic submillimeter fMRI in the human brain at 7 T: combining reduced field-of-view imaging and partially parallel acquisitions. Magn Reson Med. 2012;68(5):1506-1516.
3. Zaitsev M, Zilles K, Shah NJ. Shared k-space echo planar imaging with keyhole. Magn Reson Med. 2001;45(1):109-117.Yun S, Reske M, Vahedipour K, et al.
4. Parallel imaging acceleration of EPIK for reduced image distortions in fMRI. NeuroImage. 2013;73:135-143.
5. Porter DA, Heidemann RM. High resolution diffusion-weighted imaging using readout-segmented echo-planar imaging, parallel imaging and a two-dimensional navigator-based reacquisition. Magn Reson Med. 2009;62(2):468-475.
6. Yun S, Shah NJ. High-resolution fMRI using accelerated EPIK for enhanced characterisation of functional areas at 3T. In: Proc. Intl. Soc. Mag. Reson. Med. 23 (2015) 2468.
7. Setsompop K, Gagoski BA, Polimeni JR, et al. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med. 2012;67(5):1210-1224.
Figures