1480
Synthetic MRI of the spine using outer volume suppression and virtual coil concepts to further increase scan productivity1Global MR Applications & Workflow, GE Healthcare, Menlo Park, CA, United States, 2Department of Imaging Physics, University of Texas M.D. Anderson Cancer Center, Houston, TX, United States, 3SyntheticMR AB, Linköping, Sweden
Synopsis
Several methods for rapid simultaneous quantification of proton density, T1 and T2 maps from a single acquisition have emerged recently, allowing for retrospective synthesis of MR images with any desired contrast weighting from these maps. This work adapts a 2D fast spin echo based mapping method to spine MRI where scans are typically long and prone to artifacts. Outer volume suppression was incorporated to be able to save time by encoding only the anatomy of interest without aliasing concerns. Interleaved k-t sampling and virtual coil methods were explored to overcome limited coil acceleration capability and to further increase scan productivity.
Introduction
Techniques for simultaneous rapid mapping of proton density (PD), T1, T2, as well as B0 and B1 maps from a single acquisition enable retrospective synthesis of MR images of any desired contrast weighting based on these parametric maps [1-3]. These techniques have shown great potential for increasing scan productivity as well as maximizing the quantitative information that can be obtained from a single scan. Magnetic Resonance Image Compilation (MAGiC), a model based technique where the parameters maps are fitted to data from a 2D fast spin echo based multi echo multi saturation delay acquisition [2,4] has been successfully demonstrated in clinical applications in the brain [5]. Such advanced imaging technique could potentially benefit the area of spine MRI where exams tend to be long and artifact-prone and typically include images with at least two or more different contrast weightings (for example, T2w scans for cord pathology, T1/PD weighted scan to investigate disc pathology/ligament injury). Initial feasibility study of synthetic MRI in the spine showed challenges posed by motion, signal starvation and limited coil acceleration capability [6]. In this work outer volume suppression (OVS) was incorporated into the acquisition to be able to spatially encode only the anatomy of interest without aliasing concerns. Reconstruction strategies for improving parallel imaging performance were also explored.Method
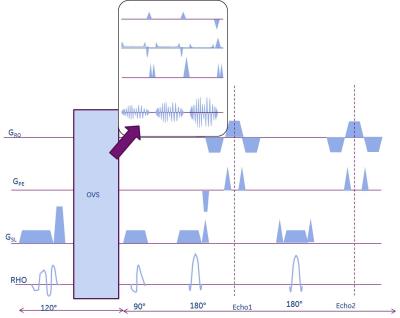
In the above mentioned MAGiC method, multiple saturation delay times and multiple spin echo times are acquired in a single 2D FSE scan [2,4]. An outer volume suppression module was incorporated into the sequence immediately before the 90° excitation to minimize outer-volume T1 recovery (Fig. 1). The OVS module consisted of three high-bandwidth (8kHz), quadratic-phase, very selective saturation (VSS) RF pulses that were cosine-modulated [7,8] to simultaneously suppress signal from either side of the phase field-of-view. 2 volunteers were scanned at the Lspine with informed consent in accordance with IRB guidelines of the site on a 3T scanner (GE MR 750 Waukesha, WI) using 4 elements of a spine array (USA Instruments) and the modified MAGiC sequence with OVS (FOV=26x20.8cm, BW=31 KHz, resolution: 1x1x4 mm3, 20 slices, scan time of 12:20 mins). Fully sampled data was acquired to allow for retrospective investigation into optimal undersampling and reconstruction strategy. K-t adaptive ARC (kat-ARC) [9] reconstruction was used on simulated staggered time shifted sampling pattern across the temporal phases. Since only 4 receive coil elements of the spine array were engaged in signal reception, the virtual coil concept which utilizes conjugate coil symmetry was used to improve parallel imaging performance [10]. T1 and T2 fitting to the reconstructed images from different delay and echo times, computation of PD from the scaling of the curves and further processing was performed by the SyMRI processing pipeline [2] (SyntheticMR AB, Linköping, Sweden).Results and Discussion
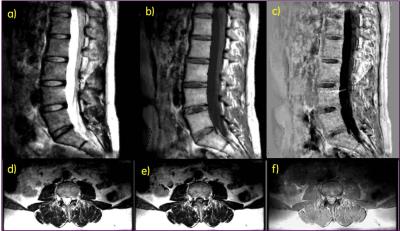
Use of OVS obviated the need for oversampling even when the PE direction was along S/I leading to about 25% saving in scan time, in spite of slight increase in TR due to specific absorption rate (SAR) constraints. Visual inspection did not show an impact of OVS on parameter maps, which confirms the assumption that OVS shouldn’t affect spins experiencing saturation recovery within the FOV. Images were reconstructed from 2X time shifted k-t undersampled data, resulting in an effective scan time of 6mins 10s. The virtual coil method reduced aliasing artifacts and noise enhancement but also introduced slight blurring in some cases. Further investigation is warranted to understand how background phase and deviation from conjugate symmetry due to T2 decay affects reconstruction performance in the virtual coil method. Representative synthetic T2w T1w and phase sensitive inversion recovery (PSIR) images in the sagittal spine and synthetic T2w, T2w fluid inversion recovery (FLAIR) and PDw images in the axial spine shown in Figure 2 demonstrate good image quality.Conclusion
Synthetic MRI with OVS technique was demonstrated in the lumbar spine with reasonable image quality for several different contrast weightings. Even though images with contrast weighting such as phase sensitive inversion recovery or double inversion recovery are typically not included in a spine protocol, such images derived from a single scan of ~ 6 mins could potentially be beneficial in gray-white matter depiction or lesion visualization. The rFOV approach employed by OVS can be beneficial for adapting synthetic MRI to deep seated anatomies such as prostate. Receive arrays with better parallel imaging performance would further improve scan productivity, and additional denoising of the reconstructed images could potentially improve the parametric mapping and consequently SNR of the derived images.Acknowledgements
NoneReferences
1. Deoni SC, Peters TM and Rutt BK e, High-Resolution T1 and T2 Mapping of the Brain in a Clinically Acceptable Time with DESPOT1 and DESPOT2, Magn Reson Med 2005; 53:237-41.
2. Warntjes JB et al, Rapid Magnetic Resonance Quantification on the Brain Optimization for Clinical Usage, Magn Reson Med 2008; 2:320-9.
3. Ma D et al, Magnetic resonance fingerprinting, Nature 2013; 495:187-92.
4. Hwang KP et al, Fat-water separation in a rapid quantitative mapping sequence, ISMRM 22nd Annual Meeting, Milan, Italy, 2014, #3201
5. Granberg , Uppman M et al, Clinical Feasibility of Synthetic MRI in Multiple Sclerosis: A Diagnostic and Volumetric Validation Study, AJNR Am J Neuroradiol. 2016;37(6):1023-9.
6. Banerjee S et al, Single Acquisition multiple contrast spine MRI using accelerated quantitative mapping, ISMRM 24th Annual Meeting, Singapore, 2016, #4393
7. Schulte R et al, Equi-ripple design of quadratic-phase RF pulses, Journal of Magnetic Resonance 2004;166: 111–122
8. Banerjee S, Han M et al, Reduced Field-Of-View Imaging with 3D Variable Flip Angle Fast Spin Echo-Feasibility in MRI of Orbits. ISMRM 23rd Annual Meeting, Toronto, Canada, 2015,#2309
9. Lai P et al, Single breathhold three-dimensional cardiac cine MRI with whole ventricular coverage and retrospective cardiac gating using kat ARC, , J Cardiovasc Magn Reson. 2012; 14(Suppl 1): W69
10. Blaimer M et al, Virtual Coil Concept for Improved Parallel MR Employing Conjugate Symmetric Signals, Magnetic Resonance in Medicine 2009; 61:93–102
Figures