1479
Rapid Spinal Cord Imaging1Radiology, Radiological Physics, University Hospital Basel, Basel, Switzerland, 2Dept. of Biomedical Engineering, University of Basel, Basel, Switzerland
Synopsis
For rapid spinal cord imaging, an inversion recovery prepared balanced steady state free precession (bSSFP) sequence with time-limited cine sampling was developed. It simultaneously acquires eight consecutive images of remarkable different tissue contrasts at 0.67mm in-plane resolution within a single measurement of only 51s per slice. The acquired images can be further combined to considerably improve the contrast to noise ratios (CNR) of the spinal cord tissues such as gray matter and white matter. Representative examples for images measured at different cervical spinal cord locations, various image combinations, and CNR gains are shown.
Purpose
In recent years, several MRI approaches have been investigated to delineate spinal cord (SC) morphology and to mitigate challenges like motion, fine structures and the low contrast between gray matter (GM) and white matter (WM) in the SC 1-3. Based on our initial and quite preliminary idea of using a cardiac relaxometry sequence for SC imaging 4, a new balanced steady state free precession (bSSFP) based approach for SC imaging with time-limited cine sampling was employed. It acquires eight consecutive images of different tissue contrasts within a single measurement of only 51s per slice. It is shown that the images can be combined to further improve the contrast to noise ratio (CNR) between the present tissues GM, WM and cerebrospinal fluid (CSF).
Methods
The suggested approach is based on an IR prepared, segmented cine-type bSSFP sequence as depicted in Fig. 1: After a non-selective adiabatic inversion of magnetization, eight sample images with the effective inversion times TI = [148.4; 210.0; 271.5; 333.1; 394.6; 456.2; 517.8; 579.3]ms are acquired in 17 shots (TRIR-IR = 3s, one dummy-shot, c.f. Fig. 1). The bSSFP sequence parameters were: TRbSSFP = 5.13ms, TE = 2.14ms, flip angle = 35deg, 12 k-space lines per segment, matrix = 192x192, FOV = 128x128mm2, in-plane resolution = 0.67x0.67mm2. Transversal slices of thickness 8mm orientated orthogonal to the course of the spinal cord were measured between the vertebrae C1-C2 and C7. The shim volume was manually selected around the particular SC location. The total acquisition time was TA = 51s per slice (no signal averaging, no triggering).
An additional sagittal overview scan was acquired: T2 weighted turbo spin echo sequence with FOV = 226x128mm2, in-plane resolution = 0.6x0.6mm2, 15 slices of thickness 3mm, TR = 3500ms, TE = 106ms
All experiments (n=11) were performed on a 3T whole-body MR system using a 64-channel phased array head and neck coil. Comparative measures for CNR were defined as CNR*tissue1-tissue2 := (<SI>tissue1 - <SI>tissue2) / STDnoise, where <SI>tissue represents the tissue signal intensity averaged over a region of interest (ROI). STDnoise refers to the standard deviation of noise, measured outside of the head.
Results
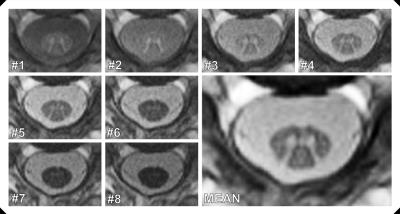
Figure 2 depicts a representative series of the eight inversion images acquired at the high in-plane resolution of 0.67mm. A remarkable tissue contrast variation can be observed. The first image with the lowest TI presents the highest CNR*GM-WM. Then, CNR*GM-WM monotonously decreases for the seven subsequent images with increasing TI (Fig. 2). Contrary, the last image has the highest CNR*WM-CSF.
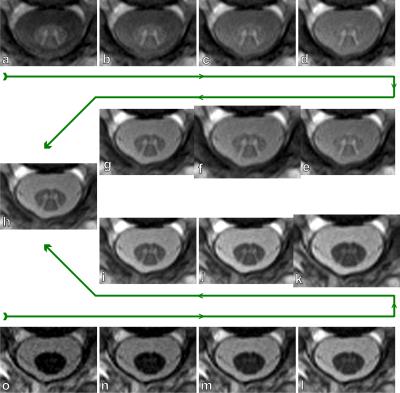
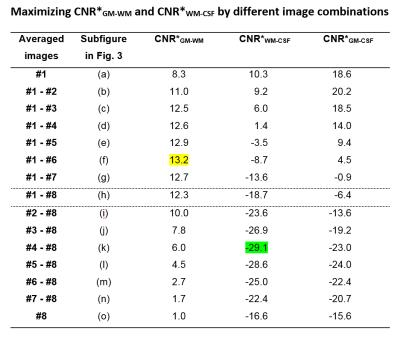
Despite the different contrasts, some of the images are combined to improve the tissues’ CNRs. A first example is already demonstrated in Fig. 2 where all eight images were averaged. The resulting mean image displays a very good contrast between CSF and WM as well as between GM and WM. The potential contrast gain by image combination is visually demonstrated in Fig. 3. Table 1 analyzes the corresponding quantitative CNR* data. An increase of CNRGM-WM by 59% compared to image #1 is achieved by combining the first six acquired images. Averaging the last five images increases CNRWM-CSF by 75% compared to image #8.
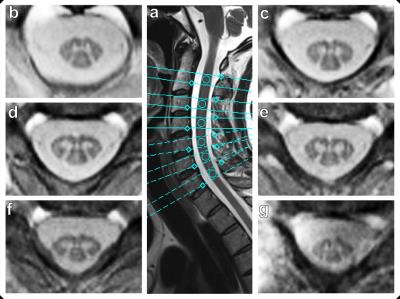
Figure 4 demonstrates the practicability of this new approach for different locations along the cervical spine.
Discussion and Conclusion
The beauty of the present approach is that it simultaneously acquires eight images of noteworthy different tissue contrast in only 51s per slice. With such a short acquisition time, any type of motion artifacts is not an issue even at the high 0.67mm in-plane resolution, as is demonstrated in Figs. 2-4. The multiple tissue contrasts at hand are of particular value for further image postprocessing like tissue segmentation and quantitations. As another benefit, the images can be combined to further improve the CNR* by 59% (GM-WM) or 75% (WM-CSF) compared to the “best” single image.Acknowledgements
This work was funded by the Swiss National Science Foundation (SNF), grant number 320030_156860.References
1. Wheeler-Kingshott CA, Stroman PW, Schwab JM et al. The current state-of-the-art of spinal cord imaging: Applications. Neuroimage 2014;84:1082-1093.
2. Stroman PW, Wheeler-Kingshott C, Bacon M et al. The current state-of-the-art of spinal cord imaging: Methods. Neuroimage 2014;84:1070-1081.
3. Papinutto N, Schlaeger R, Panara V et al. 2D phase-sensitive inversion recovery imaging to measure in vivo spinal cord gray and white matter areas in clinically feasible acquisition times. J Magn Reson Imaging 2015;42:698-708.
4. Weigel M, Bieri O. A simple and fast approach for spinal cord imaging at 3T with high in-plane resolution and good contrast. In: Proceedings Annual Meeting ISMRM 2016, Singapore, p. 4408.
Figures