1472
Early enhancement in breast DCE-MRI is sparse and can be imaged with a reduced FOV to increase temporal resolutionFederico Pineda1, Ty O Easley1, and Gregory Karczmar1
1Radiology, University of Chicago, Chicago, IL, United States
Synopsis
Early enhancement in breast DCE-MRI is very sparse, if the FOV is reduced in these images and aliasing occurs, the likelihood that two significantly enhancing voxels overlap is low. We present a method for ‘unfolding’ of aliased DCE-MRI acquisitions that closely approximates fully-sampled acquisitions. This method could be used to increase the temporal resolution of DCE-MRI at very early times when enhancement is rapidly changing, allowing for the accurate measurement of early lesion kinetics.
Purpose
The goal of this research is to increase diagnostic accuracy of breast MRI by more accurately sampling the early kinetics of contrast media uptake. During the early phase enhancement in the breast is very sparse1 and this makes it possible to acquire data with reduced field-of-view, and increased temporal resolution. Here, we tested the feasibility of a method of accelerating bilateral breast DCE-MRI by reducing the FOV and unfolding the aliased images using later un-aliased (or less aliased) images.Methods
Previous experience with an ‘ultrafast’ protocol for bilateral breast DCE-MRI (6s-10s temporal resolution) showed that the number of significantly enhancing voxels was very low in the first 30 to 45 seconds after contrast media injection1. The sparse enhancement means that if the FOV is reduced in the phase-encoding direction, allowing aliasing and reducing acquisition time, the likelihood that two significantly enhancing voxels will overlap in the aliased image is low. In a ‘proof of principle’ test, aliased images were simulated from the first 30s of full FOV acquisitions. Five cases with known enhancing lesions and moderate or marked parenchymal enhancement were selected. We selected cases with relatively dense enhancement to test how this method would work in a worst case scenario. In an initial test, an FOV of 60% the size of the full FOV was simulated. Subtractions were generated for the aliased images (post minus pre-contrast) and filtered to select significantly enhancing voxels. The correct locations (in the full FOV) of each enhancing voxel were selected based on comparison of the aliased images with fully sampled images from a later time-point; if both possible locations were enhanced in the ‘reference’ image, the voxel was copied to both. To reduce the probability of errors due to overlapping voxels in aliased images, we tested a progressive unfolding approach. A (simulated) small FOV was used for the first time point when enhancement was very sparse, and the FOV was progressively increased at the 2nd and 3rd time-points, so that the same enhancing voxels could not overlap in subsequent images. We simulated FOVs of 31%, 44% and 77% of the full FOV. To unfold the aliased images, they were first filtered as described above. Then, comparison of early, highly aliased images, with later, less aliased images helped to identify the true locations of enhancing voxels. Voxels that aliased onto each other at a specific time point were resolved by linearly interpolating the signal value from the preceding and following time-points (‘acquired’ with different FOV’s) (see diagram in Fig. 1). Unfolded and original images were compared using the complex wavelet structure similarity index measure (SSIM)2.Results
In the initial aliasing simulations (Fig. 2), an average of 2.2% of the enhancing voxels above the chest wall overlapped in the aliased images (range 0% - 9.8%). While this number was low, overlapping voxels were artificially brighter in the unfolded images. The SSIM numbers were 0.48, 0.73, and 0.77 for each aliased time-point (numbers closer to 1 indicate very similar images). For the progressive aliasing tests (Figs. 3 and 4), an average of 2.5% of the enhancing voxels above the chest wall overlapped (range 0% - 6.6%). SSIM values for the unfolded images were 0.64, 0.93, and 0.97 for each time-point.Discussion
These simulations show that it is possible to unfold aliased breast DCE-MRI images to produce un-aliased images that closely approximate fully-sampled images. The progressive aliasing and unfolding approach estimates the signal value of overlapping voxels. Progressive unfolding allows high temporal resolution imaging during the early phase of contrast media uptake when enhancement is sparse, and measurement of rapidly changing enhancement can improve diagnostic accuracy. Temporal resolution is lower, and the FOV is more fully sampled at later times, as enhancement becomes denser and changes slowly. All enhancing lesions were accurately recovered in the simulations. Images generated by this algorithm were very similar to the original images. Some artifacts were present in earlier images ‘acquired’ with smaller FOV, but further refinement of the method will reduce artifacts. Future work will use information from neighboring voxels to refine the estimates for overlapping areas. This approach does not rely on specialized acquisition techniques.Conclusion
‘Progressive Unfolding’ allows accelerated bilateral breast DCE-MRI during the early contrast media uptake phase. This method relies on the geometry of axial breast MRI and the fact that early enhancement in the breast is sparse. Progressive aliasing and unfolding led to images that were very similar to fully-sampled images, and that could be acquired with a temporal resolution as low as 2s.Acknowledgements
No acknowledgement found.References
1. Pineda FD, Medved M, Wang S, et al. Ultrafast bilateral DCE-MRI of the breast with conventional Fourier sampling: preliminary evaluation of semi-quantitative analysis. Acad Radiol. 2016;23(9):1137-1144. 2. Wang Z, Bovik AC, Sheikh HR, Simoncelli EP. Translation insensitive image similarity in complex wavelet domain. Proc. IEEE Int. Conf. Accoust., Speech, and Signal Processing, Philadelphia, PA. 2005.Figures
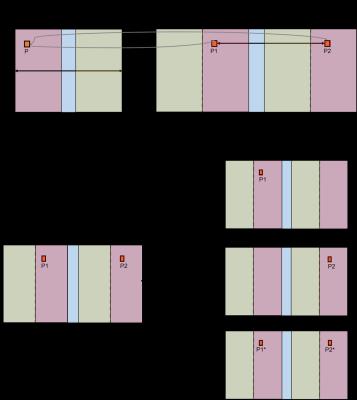
Diagram of ‘unfolding’ methods. a) a first approximation is
created by replicating the aliased portions of the image. b) the unfolded image
is compared to later reference images acquired with a larger or fully-sampled
FOV, if only one of the potential locations is enhanced (‘on’) in the later
images, the voxel is copied to that location and its alias is zeroed out; if
both locations are ‘on’ an estimate is created for each voxel’s intensity (in
the progressive case) or it is copied to both locations.
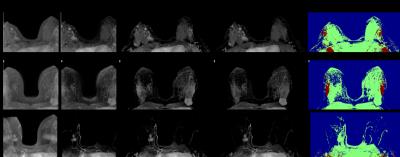
Maximum intensity projections (MIPs) for three cases at the
third-timepoint (18-25s) after contrast enhancement in the aorta: A) Simulated
aliased dynamic image; B) Aliased subtractions (post minus pre contrast); C)
‘Unfolded’ subtraction images; D) Original subtraction images; E) Key-image
showing enhancing voxels that did not overlap in green, and those that did in
red, because these are projections, if a voxel overlapped in even one slice, it
is shown as red in the key image.
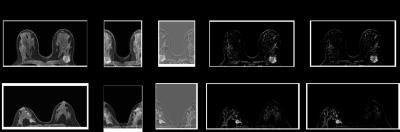
Example
slices showing enhancing lesion from cases with a simulated FOV of 44% of the
original size: A) original dynamic image; B) aliased dynamic image; C) aliased
post minus pre-contrast subtraction image; D) unfolded subtraction at full FOV;
E) original subtraction image.
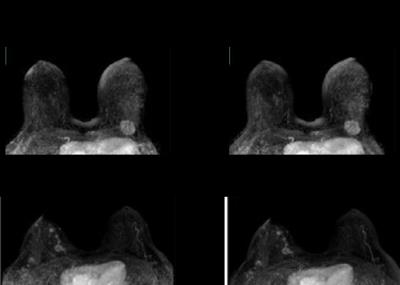
Maximum intensity projections of unfolded images (A)
and original post minus pre-contrast subtraction images (B) for time-points with a
simulated FOV of 44% of the fully sampled FOV.