1462
AWESOME-Based De-Noising of Complex-Valued fMRI Time Series1NMR, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 2Section on Functional Imaging Methods, National Institute of Mental Health, Bethesda, MD, United States
Synopsis
In this study we investigate possible benefits of an application of ‘AWESOME’ de-noising on fMRI. The application in a high-SNR finger tapping experiment showed a reduction of the already low thermal noise contribution and therefore improvement of tSNR and reduction of false positives; no adverse effects in the form of smoothing or suppression of ‘true’ activation was observed. A second investigation of the scalability of tSNR improvement on a resting state experiment with variable slice thickness / SNR showed that thermal noise can be reliably reduced and the tSNR proportionally improved without visible reduction of detail sharpness / resolution.
Purpose
Recently, a de-noising technique referred to as ‘AWESOME’ has been proposed that reduces noise by ‘averaging’ of complex MRI data in wavelet space.1 It permits to use an image series acquired with varying contrast as input without the need for repeated acquisitions. Application to relaxation studies1 and diffusion-weighted MRI2 experiments demonstrated that quantitative information is well preserved in the de-noised images while object features initially covered by noise are regained. Here, we investigate a novel application of the AWESOME algorithm to de-noise high-resolution 3D gradient-echo (GRE) EPI and 3D VASO time series used for BOLD and cerebral blood volume (CBV) weighted fMRI.3-6Methods
Acquisition: Two data sets acquired at 7T (MAGNETOM 7T, Siemens Healthcare, Germany) with a 32-channel head coil (Nova Medical, Wilmington, MA, USA) were analyzed. (i) resting-state BOLD-based fMRI data consisting of 3D GRE-EPI scans in a healthy subject employing the following parameters: 1.5x1.5mm2 in-plane resolution, varying slice thickness of 0.25/0.5/1/2 mm, 12 slices, TR=3s, TE=22ms, in-plane GRAPPA 2, 50 repetitions.3 (ii) task-based fMRI data: CBV and BOLD contrasts were recorded using a 3D slice-saturation slab-inversion VASO (SS-SI-VASO) sequence: 0.75x0.75mm2 in-plane resolution, 1.8mm slice thickness, 8 slices, TR=3s, TE=22ms, in-plane GRAPPA 2.3 The paradigm was a unilateral finger-tapping task (block design; 12-min total acquisition time).
Preprocessing: Data were corrected for motion. Subsequently, the background phase in the data was corrected using an algorithm based on total variation de-noising.7 The AWESOME algorithm requires a normalized noise-level map that was calculated from the highest frequency band of the 1D wavelet transformation of the time line for each voxel.
AWESOME de-noising: AWESOME1,2 operates in the wavelet space of complex MR images. In the wavelet space, signals (i.e. brain structures) and noise are well separated in multiple frequency bands, with thermal noise mostly represented in high frequency bands. From the complex noise distribution, a filter is derived to separate noisy and mostly meaningful data in the wavelet space. A mean wavelet data set is calculated over the image series. From this high SNR mean data set, the original signal contributions are estimated based on phase-weighted rescaling using the fraction of total signal energy per voxel over the series and each single voxel signal in the wavelet space. The thereby estimated signals replace noisy signals in the original wavelet data. The inverse wavelet transformation of the new data results in a de-noised MR image series.
The calculation of the mean wavelet data set was performed differently in both time series, because data containing alternating contrasts (i.e. CBV-weighted VASO and BOLD contrasts acquired with the SS-SI-VASO sequence) can suffer from cancellation of meaningful data in the complex mean. Thus, (i) the mean of rsMRI data is calculated from the complex wavelet coefficients; (ii) the mean of the task-based-fMRI data is calculated from the mean imaginary part and the mean absolute real part, which is then bias-corrected for the background noise.
FSL was used for statistical fMRI analysis and estimation of smoothness in the VASO data set.
Results
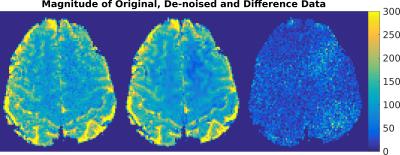
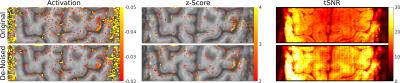
rs-fMRI: In Figure 1, the result of AWESOME-based de-noising is demonstrated for the acquisition with the minimal slice thickness (0.25 mm). De-noising of rsMRI data resulted in an SNR improvement per volume by up to four times as compared to the original SNR. As a consequence, the time-series SNR (tSNR) is almost doubled (Figure 2). Most notably, this is obtained without visible loss of image detail. The results for all rs-fMRI data are summarized in Figure 2.
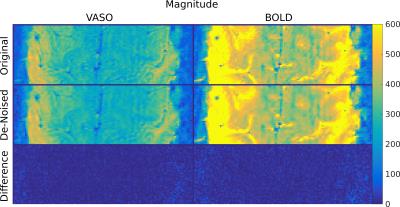
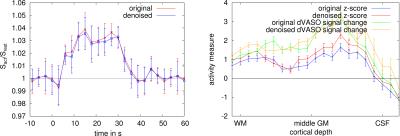
Task-based fMRI: Although the voxel size was rather small, the SNR was already relatively high. Therefore, the effect of de-noising is not readily visible in Figure 3. Yet, fMRI analysis showed a reduction of false-positives in the statistical maps of the VASO signal changes (Figure 4). On the other hand, the areas of activation appear unchanged (cf. Figure 4). This can be seen as well in the VASO signal time course (Figure 5). Also, the corresponding cortical profiles of VASO signal change are not significantly altered.
Discussion & Conclusion
The multi-contrast data suggest that the de-noising preserves the temporal and spatial signature of “true” activation while reducing false positives. In conclusion, application of AWESOME to fMRI may offer the potential to produce meaningful results from lower SNR data, such as acquisitions at very high resolutions or with reduced paradigm length.Acknowledgements
This work was funded through the Helmholtz Alliance ICEMED. Laurentius Huber is supported by the American NIMH Intramural Research Program.References
1. Marschner H, Pampel A, Möller HE. Adaptive Averaging of Non-Identical Image Series in the Wavelet Space. Proc Intl Soc Mag Reson Med 2015;23:3721.
2. Marschner H, Eichner C, Anwander A, Pampel A, Möller HE. De-noising of diffusion-weighted MRI data by averaging of inconsistent input data in wavelet space. Proc Intl Soc Mag Reson Med 2016;24:2071.
3. Huber L, Ivanov D, Marrett S, Panwar P, Uludag K, Bandettini PA, Poser BA. Blood volume fMRI with 3D-EPI-VASO: any benefits over SMS-VASO? Proc Intl Soc Mag Reson Med 2016;24:0944.
4. Huber L, Ivanov D, Krieger SN, Streicher MN, Mildner T, Poser BA, Moller HE, Turner R. Slab-selective, BOLD-corrected VASO at 7 Tesla provides measures of cerebral blood volume reactivity with high signal-to-noise ratio. Magn Reson Med 2014;72(1):137-148.
5. Huber L, Goense J, Kennerley AJ, Trampel R, Guidi M, Reimer E, Ivanov D, Neef N, Gauthier CJ, Turner R, Moller HE. Cortical lamina-dependent blood volume changes in human brain at 7 T. Neuroimage 2015;107:23-33.
6. Krieger SN, Huber L, Poser BA, Turner R, Egan GF. Simultaneous acquisition of cerebral blood volume-, blood flow-, and blood oxygenation-weighted MRI signals at ultra-high magnetic field. Magn Reson Med 2015;74(2):513-517.
7. Eichner C, Cauley SF, Cohen-Adad J, Möller HE, Turner R, Setsompop K, Wald LL. Real diffusion-weighted MRI enabling true signal averaging and increased diffusion contrast. NeuroImage 2015;122:373-384.
Figures