1454
Validation of CSF based calibration for accurate and robust quantification of water content.1Medical Imaging Physics, Institute of Neuroscience and Medicine, Juelich, Germany, 2Faculty of Medicine, RWTH Aachen, JARA, Aachen, Germany
Synopsis
Estimating tissue water content is challenging. Reliable quantification of the water content requires significant number of corrections and calibration to a reference. In this work, we proposed to use a region in cerebrospinal fluid for robust calibration; this is further validated in a cohort of healthy volunteers and compared to existing methods.
Purpose
Water
content imaging of soft tissue is an essential element in the quest to make MRI
quantitative. Accurate and precise estimation of water content in vivo
using MR has shown to be relevant in various pathologies to gain exact
information about the type of damage and its extent.1,2,3 However,
reliable quantification of the water content requires a significant number of
corrections4,5 and calibration to a reference. Recently, a robust
calibration method has been proposed which utilizes ventricular cerebrospinal
fluid (CSF) as a reference.5,6 In this study, this concept is
further exploited to show reliable calibration.Methods
MR Data Acquisitions. MR measurements were performed on a 3 Tesla Siemens (Erlangen, Germany) TIM TRIO scanner with a body coil for radiofrequency (RF) excitation whereas a 32-channel receive phased array head coil was used for signal reception. Multi-echo Gradient Recalled Echo (GRE) MRI scan (TR=1800ms, TE=5.6ms and FA=40°) allows one to estimate the MR-visible water content. However, it requires the following corrections: i) compensation of the transmit (B1Tx) and receive field (B1Rx) inhomogeneity; ii) compensation of the T2* decay; iii) correction of the T1-saturation effect; iv) correction of residual nonuniformity.4,5 The corrections i) to iii) involve the inclusion of additional MRI acquisitions to the water content imaging protocol, namely, a second GRE scan (TR=500ms, TE=5.6ms and FA=90°) for estimating longitudinal relaxation time (T1), a series of four single-shot GRE-EPI measurements (TE=11ms and FA=30°/60°/90°/120°) for estimation of B1Tx and a set of two low-resolution GRE scans (TR=500ms, TE=5.6ms and FA=40°) for obtaining B1Rx map.5
Simulations. Error propagation analysis using Monte-Carlo simulations was performed to observe the effect of noise standard deviation at σ = 6 x 10-3 x M0, (M0 is water content contrast) corresponding to the actual SNR values obtained from a 3 Tesla scanner. Signals for GRE acquisitions were simulated and used the same T1 and M0 estimation algorithms as for processing in vivo MRI data.
In vivo experiments. 28 healthy participants (age 26.1±2.7 years, male only) were recruited following agreement through written, informed consent for participation in the study.
Calibration: Method-1. The CSF signal was used for quantification of water content and was estimated as following: i) the entire right and left ventricles were segmented using T1 and T2*: T1 > 2.9s and T2* > 1.5s; denoted by V ii) further partitioned into sub-regions (Vi)0<i<n of constant B1Tx values (practically ΔB1Tx/B1Tx <0.5); and subsequently estimated and computed the weighted average using following.6
Calibration: Method-2. Ventricle volumes V using “Method-1” were registered to the MNI-152 brain atlas using FSL-FLIRT, 7 then integrated and normalized to produce voxel-wise heat map (Figure.2). Voxels from the heat map with an intensity ≥15% were regarded as calibration criteria. Water content estimation from seventeen ROIs of the MNI structural atlas was performed and compared statistically using pair-wise t-test.
Results
Monte-Carlo simulations show the bias and the standard deviation in the estimation of T1 as a function of the ground truth T1 and of M0. The bias bT1 as well as bM0 for the estimation of T1 and M0 are increasing with increasing T1 values. Analyses for the measurement obtained are reported in Table 1. A heat map overlaid on a MNI template in three orthogonal planes is shown in Figure 2. T1 and water content values were accessed using both methods. ROI analysis was performed and compared statistically and to literature values; results are listed in Table 2.Discussion
Robust calibration is extremely important to determine intra-subject results. Heat maps can provide the possibility to quantify water content contrast image. This allows reliable comparison of the water content maps produced by means of different techniques without a priori knowledge of T1 and T2*. Statistical analysis verifies the precision of the voxels selected for calibration and confirms the similar distributions. The inter-subject variability as measured by the standard deviation in both methods is low; suggesting that the region delineated using a heat map is practicable method.Conclusion
A calibration technique for water content mapping was optimized based on simulations and analytical data. We propose a new method utilizing an automatic calibration technique for the quantification of water content images. Based on these results, the proposed methodology can thus be used for the precise and accurate estimation of in vivo cerebral water content despite different water content estimation techniques.Acknowledgements
No acknowledgement found.References
[1] Shah et al. NeuroImage 41, 706-717, 2008.
[2] Reddy et al. AJNR 30,978-984, 2009.
[3] Reetz-Abbas et al. PLOS-One 2015.
[4] Neeb et al NeuroImage 42, 1094–1109, 2008.
[5] Abbas et al. MRM 2014.
[6] Abbas et al. NeuroImage 2015.
[7] Andersson, J.L.R., and Sotiropoulos, S.N. NeuroImage 2016, 125:1063-1078.
[8] Cheng H-LM et al. Magn Reson Med 2006;55:566–574.
[9] Stanisz GJ et al. Magn Reson Med 2005;54:507–512.
[10] Ethofer T et al. Magn Reson Med 2003;50:1296–1301.
[11] Gelman N et al. Magn Reson Med 2001;45:71–79.
[12] Volz S et al. Neuroimage 2012;63:540–552.
[13] Whittall KP et al. Magn Reson Med 1997;37:34–43.
[14] Warntjes J et al. Magn Reson Med 2007;57:528–537.
[15] Oros-Peusquens A-M et al. Magn Reson Mater Phys 2008;21:131–147.
[16] Preibisch C et al. Magn Reson Med 2009;61:125–135.
Figures
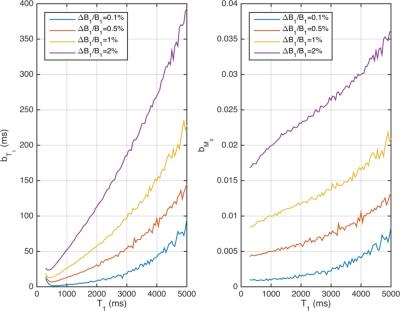
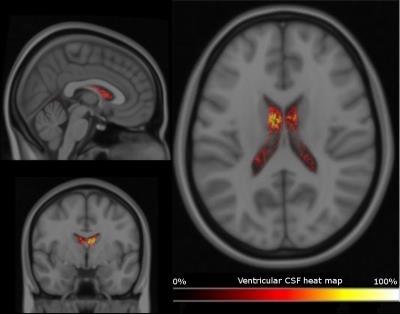
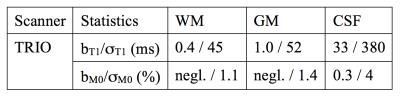
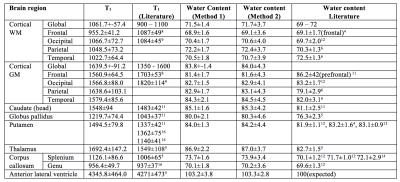
Table 2. Estimated T1, water content values and corresponding literature values in white matter / grey matter / CSF regions of the in vivo brain. Statistical analyses (using pair-wise t-test) revealed no significant differences between water content values estimated using both methods.