1440
A fully automatic prostate segmentation method for both DWI and T2WI1Academy for Advanced Interdisciplinary Studies, Peking University, Beijing, People's Republic of China, 2Department of Radiology, Peking University First Hospital, Beijing, People's Republic of China, 3Philips Healthcare, Suzhou, People's Republic of China, 4College of Engineering, Peking University, Beijing, People's Republic of China
Synopsis
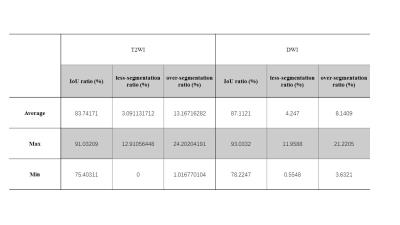
Automatic prostate segmentation in MR images is a meaningful work, not only can be used in the first step of the Prostate Imaging Reporting and Data System, but also helps to predict pathologic stage of disease by determining the prostate volume. Here we show a novel new method can get the prostate contour for both T2WI and DWI fast and accurately without any manual intervention. The segmentation accuracies for 60 images are 83.7% (T2WI) and 87.1% (DWI). Even in some cases, such as prostate hyperplasia, our method shows good robustness.
Introduction
Prostate cancer is one of the most common malignant cancers for men [1], and the MRI is the most common used non-intrusive technique to diagnosis prostate cancer [2]. However, the diagnosis depends on the personal experience of the radiologist who could be trained very differently. The Prostate Imaging Reporting and Data System (PI-RADS) [3] is the most likely procedure to solve this problem, and the MR image segment is the first step for PI-RADS. What is more, finding the contour of prostate is very useful to determine prostate volume (PV), and can help with facilitates an assessment of prostate disorders.
However, prostate segmentation in MRI image is a challenging task due to low signal-to-noise ratio, small size of prostate and complex prostate anatomy. And the patient who needs a MRI to diagnose always have some pathological changes of prostate, which make prostate hard to recognize in MR image.
Method
A. Image Acquisitions
Clinical
routine MRI was conducted on a cohort of 30 subjects, including healthy subjects,
prostate cancer patients and prostate hyperplasia patients. The
imaging was performed on a 3.0 T Ingenia system (Philips Healthcare, the
Netherlands), with acquisition of T2-weighted, diffusion-weighted and dynamic
contrast-enhanced images, with standard imaging protocol.
For each subjects, three typical slices both in
DWI and T2WI were selected as the data sets .
B. Segmentation Overview
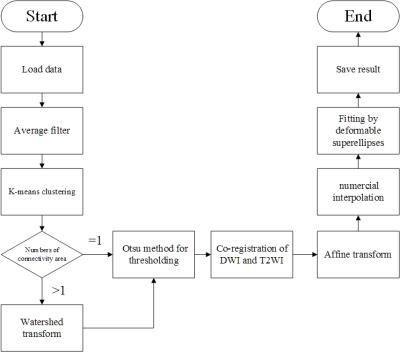
The proposed prostate segmentation method is divided into seven steps (Fig.1).
Step 1: An average filter was used to denoise in the images of DWI.
Step 2: K-means algorithm adopted for gray value.
Step 3: Watershed transform was used to pick out adhesion part such as bladder and urethra which the gray value sometimes is similar to the prostate areas.
Step 4: Got a binary image with OTSU method.
Step 5: DWI and T2WI image was co-registrated with each other to give possibility of higher accuracy.
Step 6: Let the binary image have a affine transform with the parameter gained in Step 5.
Step 7: We make the binary image outline fitting with deformable superellipses model.
C. The deformable superellipses
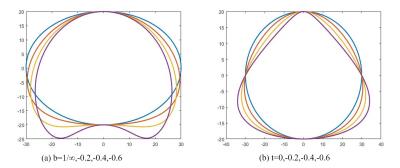
Superellipses is also called Lamé curve, it is an extension of the elliptic equation by changing some parameters. Gong LX et al. proposed a method using deformable superellipses in TRUS images segmentation as a priori knowledge[4].We find this model can also be applied to fit the boundary of binary image. Fig.2 and the formulas as follows show a dynamic process from a standard ellipse to a deformable superellipses by change eight parameters(ax,ay,e,lx,ly,r,t,b).
The parametric form of standard ellipse:$$ \begin{cases}x(\theta) = a_{x}\cos^e\theta\\ y(\theta) = a_{y}\sin^e\theta & \end{cases} -\pi\leq\theta\leq\pi $$
Translation:$$\begin{cases}x_{1}=x+l_{x}\\y_{1}=y+l_{y}\end{cases}$$
Rotation:$$ \begin{cases}x_{2} = x_{1}\cos(r)-y_{1}\sin(r)\\ y_{2} = y_{1}\sin(r)+x_{1}\cos(r)\end{cases}$$
Tapering:$$ \begin{cases}x_{3} = (\frac{t\times y_{2}}{a_{y}}+1)x_{2}\\ y_{3} = y_{2}\end{cases} -1\leq t \leq1$$
Bending:$$ \begin{cases}x_{4} = (\frac{a_{y}}{b}-y_{3})*\sin(\frac{x}{\frac{a_{y}}{b}-y_{3}})\\ y_{4} = \frac{a_{y}}{b}- (\frac{a_{y}}{b}-y_{3})*\cos(\frac{x}{\frac{a_{y}}{b}-y_{3}})\end{cases} -1\leq b\leq1(b\neq0) $$
Result
A. Accuracy in Our In-House Images:
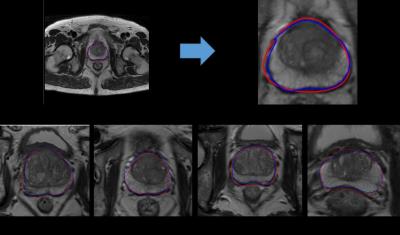
The first row in Fig. 2 shows an examples of segmentation result in T2WI image, where (a) is a complete T2WI image and (b) is the local magnification of (a). the second row in Fig.3 from (c) to (f) shows others segmentation result after magnification. All manual segmentations from our datasets were outlined by two experts with more than five-year-experience.
We evaluated our algorithm between the computer generate of the prostate boundaries and the manual outlinings on the same image by intersection-over-union(IoU), over-segmentation ratio and less-segmentation ratio. The results show in Tab.1 and Fig. 3 indicate that the proposed method achieved a good segmentation accuracy for the whole prostate gland.
B. Computational Time:
The mean segmentation time of the implemented algorithm for one MR image typically takes less than 4 seconds on a Core i5 PC running at 3.2 GHz (using MATLAB).
Discussion
In this paper, we describe and evaluate a new method to segment the prostate gland automatically from both DWI and T2WI. We verified the feasibility of using deformable superellipses model to fitting the edges in MR images. In our study, by using the deformable superellipses to fit the boundary, the results are similar to the results of the experts. Even in some special cases, some cases like Fig.3(d) and Fig.3 (f), the glandular organ of prostate gland adheres to the surrounding tissue, our algorithm can still get relatively good results.
On the other hands, the shape of the superellipses is symmetrical, so when some patients with severe lesions that the shape of the prostate have been greatly deformed , the accuracy of segmentation will be decreased.
Acknowledgements
No acknowledgement found.References
[1]Galbraith S M, Duchesne G M. Clinical Oncology Update: Prostate Cancer Androgens and Prostate Cancer: Biology, Pathology and Hormonal Therapy[J].
[2]Villers A, Lemaitre L, Haffner J, et al. Current status of MRI for the diagnosis, staging and prognosis of prostate cancer: implications for focal therapy and active surveillance[J]. Current opinion in urology, 2009, 19(3): 274-282.
[3]Roethke M, Blondin D, Schlemmer H P, et al. PI-RADS classification: structured reporting for MRI of the prostate[J]. Rofo, 2013, 185(3): 253-261.
[4]Gong L, Pathak S D, Haynor D R, et al. Parametric shape modeling using deformable superellipses for prostate segmentation[J]. IEEE transactions on medical imaging, 2004, 23(3): 340-349.
Figures