1435
Improved Infant MRI Brain Extraction utilizing Clustering and Morphological Approaches1The Developing Brain Research Laboratory, Children’s National Health System, Washington, DC, United States
Synopsis
Accurate brain extraction is a key procedure in neuroimage analyses. This paper aims to solve the intracranial cavity overestimation issue inherent to existing brain extraction methods when applied to
Purpose
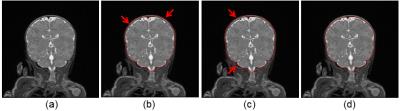
Brain extraction in MR images is an essential step in quantifying brain development and its accuracy critically impacts subsequent brain analyses. One of the common issues with brain extraction methods is that the extracted intracranial cavity (ICC) is larger than the ground truth. For example, Gui et al.’s method1 based on marker-based watershed algorithm works effectively if the gap between the skull and ICC is obvious in T2-weighted MR images. However, for many neonatal brain MR studies, the distance between the skull and ICC is often very small (Figure 1a); this leads to overestimation of the ICC masks (Figure 1b, 1c). To solve this issue, we combined k-means clustering method2 and morphological approaches to improve ICC extraction in these methods.Methods
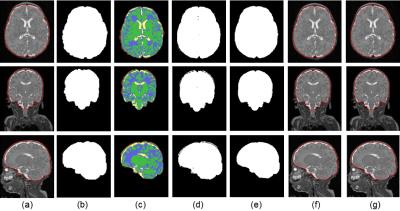
We used T2-weighted MR studies acquired on GE 3T DISCOVERY MR750 (echo/repetition time=64.74/2500 ms; flip angle=90º; voxel size=0.625×0.625×1 mm3) from preterm infants born < 32 weeks gestational age (GA) and weighing < 1500 grams at birth. Exclusion criteria included structural brain abnormalities in MR image. The study was approved by the institutional review board and parental consent was obtained. Infant brain was extracted according to the following pipeline: 1) N4 filtering for bias field correction; 2) radiological reorientation; 3) resampling of T2 images to obtain isotropic voxels; 4) obtaining a first ICC using an available brain extraction method (Figure 2a); 5) k-means clustering2 to classify the T2 intensities in the extracted ICC mask into 4 labels (Figure 2c): white matter (WM, green in Figure 2c), grey matter (GM, blue in Figure 2c), cerebrospinal fluid (CSF, yellow in Figure 2c), and other tissues (red in Figure 2c); 6) removing the class labeled “other tissues” and binarizing the image (Figure 2d); 7) eroding the binary mask in Figure 2d; 8) taking the largest connected component in the binary mask; 9) dilating the binary mask; 10) performing a closing operation of the binary mask; 11) filling holes in the binary mask (Figure 2e); and 12) resampling the binary mask back to the original T2 image. A ball-shaped structuring element with a radius of 4 voxels was used in all morphological operations. Note that, if the voxels in the T2 image are isotropic, steps 3 and 12 are skipped.Results
We evaluated brain MR images from 28 preterm infants (GA at birth, mean±SD=28.3±2.4 weeks; birth weight, mean±SD=976±318 grams). MR studies were performed between 37 to 44 weeks GA (mean±SD=40.6±1.7). The Dice ratio was used to measure the brain extraction accuracy by comparing automatically extracted ICC with manually segmented ICC (i.e., ‘ground truth’). The mean±SD Dice ratios of 28 subjects were 96.78% ±1.12% by Gui et al.1, 95.04%±2.55% by BET23, and 94.14%±0.58% by iBEAT4. The proposed method achieved 1.20% (paired t-test; p=1.2e-05), 3.04% (p=8.2e-07), and 2.66% (p=1.1e-16) higher mean Dice ratios than Gui et al.1, BET23, and iBEAT4, respectively. The standard deviation of Dice in all three methods was reduced (Table 1) demonstrating the effectiveness of the proposed method for infant brain extraction.Discussion and Conclusion
The proposed method effectively solves the problem of ICC overestimation in existing brain extraction methods. Taking advantage of the lower T2 intensities of the region between the skull and the brain tissues when compared to the rest of the brain, we used a clustering method to effectively identify and remove this darker region. Considering there are some brain tissues inside the ICC with low T2 intensities, such as veins and myelinated white matter, we further use mathematical morphology operations such as image closing and hole filling to cover those regions. Note that, if there are large regions of myelinated white matter in the inferior part of the brainstem, the proposed method might misclassify these regions. However, this phenomenon is rarely seen and was not observed in our 28 preterm infants. In summary, the experimental results show the robustness and effectiveness of the proposed method for infant brain extraction compared with state of the art methods such as Gui et al.1, BET23, and iBEAT4.Acknowledgements
No acknowledgement found.References
1. Gui L, Lisowski R, Faundez T, et al. Morphology driven automatic segmentation of MR images of the neonatal brain. Med Image Anal. 2012;16(8):1565–1579.
2. Hartigan J A, Wong M A. Algorithm AS 136: A k-means clustering algorithm. Appl Stat. 1979;28(1):100-108.
3. Jenkinson M, Pechaud M, and Smith S. BET2: MR-based estimation of brain, skull and scalp surfaces. In Eleventh Annual Meeting of the Organization for Human Brain Mapping, 2005;17:167.
4. Dai Y, Shi F, Wang L. iBEAT: a toolbox for infant brain magnetic resonance image processing. Neuroinformatics. 2013;11(2):211-225.
Figures