1426
Flexible Prospective Compressed Sensing Acceleration of Prostate DCE-MRI with Quantized CIRCUS1Biomedical Translational Imaging Centre (BIOTIC), Nova Scotia Health Authority, Halifax, NS, Canada, 2Diagnostic Radiology, Dalhousie University, Halifax, NS, Canada, 3Physics and Atmospheric Science, Dalhousie University, Halifax, NS, Canada, 4Biomedical Engineering, Dalhousie University, Halifax, NS, Canada
Synopsis
Improving the temporal resolution of dynamic contrast-enhanced (DCE) MRI sequences often requires a reduction in image spatial resolution or quality. We propose an acquisition and reconstruction strategy, Quantized CIRCUS, which allows reconstruction of prospectively accelerated DCE-MRI data with desired spatial and temporal resolution, similar to golden-angle radial acquisition schemes but using Cartesian sampling. We demonstrate that this approach allows improved temporal resolution compared to standard clinical methods, without significant degradation of image quality or resolution, which may provide more accurate information for diagnosis of diseases like prostate cancer.
Purpose
Accelerated dynamic contrast-enhanced (DCE) MRI is an area of significant interest, since increased temporal resolution may permit more accurate assessment of pharmacokinetics and diagnosis of tissue pathologies such as cancer. However, the degree of undersampling that can be achieved without significant loss of image quality or time course fidelity cannot always be determined a priori, and prescribing an acceleration factor that produces high temporal resolution may result in poor image quality or vice-versa. This has led to strategies such as golden angle radial sampling (GRASP1) in which arbitrary numbers of radial lines can be retrospectively combined without significant overlap. In this work we demonstrate a related approach based on CIRcular Cartesian UnderSampling (CIRCUS2) that uses combinations of rudimentary sampling ‘quanta’ to allow reconstruction of images at multiple effective undersampling rates.Methods
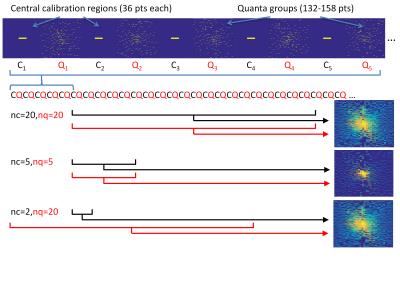
CIRCUS involves sampling points along nested squares, with spacing between points on the same square governed by the golden ratio. For an NxN square matrix, the smallest possible sampling pattern (quantum) is a single radial line of N/2 points. Two user-specified parameters introduce shear or twist to these quanta, changing the incoherence of the sampling. Our approach involves collecting large numbers of successive quanta and combining them retrospectively to achieve desired undersampling factors (see Figure 1). For a given matrix size and shear/twist parameters, the undersampling pattern is deterministic and is easily computed during scan prescription. The central 6x6 points in k-space are fully sampled at regular intervals to help stabilize the reconstruction, which was performed using the pics command of the BART toolbox3.
Images were acquired on a 3T MR750 system (GE Healthcare, Waukesha WI) with a 32-channel RF coil. Research protocols were approved by the local REB. Two subjects undergoing a clinically indicated prostate MRI exam were prospectively recruited, and returned shortly after their clinical exam for a second scan session in which accelerated DCE-CIRCUS data was collected. Acquisition parameters were: 224x192x30 matrix, 340x340x90mm FOV, TR=4ms, FA=12, 2 echoes for fat-water separation with FLEX. 17 temporal phases were acquired, each consisting of 200 unique quanta and 20 fully-sampled central regions. The time between central regions (i.e. the best possible temporal resolution that can be reconstructed) was 750ms and the time for each phase was 15s, for a total scan time of 4:15. Parameter-matched DCE-DISCO4 images from the clinical exam were used for comparison; this series had 60 temporal phases collected at an effective temporal resolution of 3.6 seconds after view sharing (total scan time 3:36). All reconstructed CIRCUS images were registered as closely as possible to the first volume of the DISCO series. Pharmacokinetic parameter mapping of all reconstructed time series was performed with GenIQ (GE Healthcare).Results and Discussion
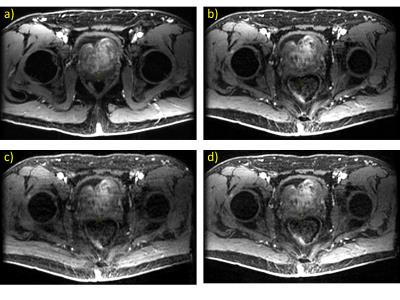
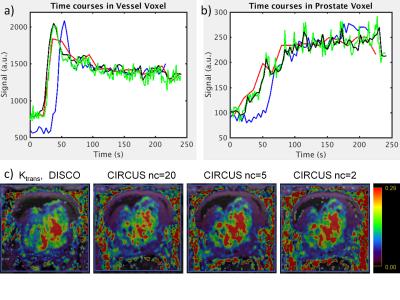
Figure 2 shows representative individual images from DISCO and CIRCUS time series. Image quality is influenced primarily by the number of quanta used to sample the periphery of k-space. Small fat-water separation artifacts sometimes appear when using small numbers of calibration regions (data not shown) which may impose a lower limit on the achievable temporal resolution. Figure 3 shows time courses from representative voxels, confirming that using fewer central regions improves temporal resolution, up to 2X that of DISCO with nc=2. Computed pharmacokinetic maps (e.g. Ktrans in Figure 3c) also appear similar; further analysis and simulation will be needed to evaluate what additional diagnostic information is provided by the increased temporal resolution of this method, and to assess how the number of peripheral quanta impacts temporal behavior in smaller structures.
The quantized CIRCUS acquisition scheme allows straightforward combination of different numbers of central regions and peripheral quanta to optimize spatial and temporal quality for a given application. This is possible in radial imaging only by enforcing data fidelity over a limited number of spokes [1]. In the data shown, each volume in the time series was reconstructed separately, but processing all acquired data simultaneously may further improve performance by allowing more appropriate regularization or model-based reconstructions of the entire time course5.
Conclusions
Quantized CIRCUS permits flexible retrospective reconstruction of DCE-MRI data similar to radial approaches but with a Cartesian sampling strategy. The temporal resolution of DCE-MRI data can be significantly improved compared to standard protocols while retaining comparable image quality.Acknowledgements
Funding for this research was provided by the Atlantic Innovation Fund, an Investigator Sponsored Research Agreement with GE Healthcare, and the NSERC Discovery Grant program (SDB).
References
1.Feng L, Grimm R, Block KT, Chandarana H, Kim S, Xu J, Axel L, Sodickson D, Otazo R. Golden-Angle Radial Sparse Parallel MRI: Combination of Compressed Sensing, Parallel Imaging, and Golden-Angle Radial Sampling for Fast and Flexible Dynamic Volumetric MRI. Magn Reson Med 2014;72:707–717.
2. Liu J and Saloner D. Accelerated MRI with CIRcular Cartesian UnderSampling (CIRCUS): a variable density Cartesian sampling strategy for compressed sensing and parallel imaging. Quant Imaging Med Surg 2014;4(1):57-67.
3. Uecker M, Ong F, Tamir JI, Bahri D, Virtue P, Cheng JY, Zhang T, Lustig M. Berkeley Advanced Reconstruction Toolbox. Proc. Intl. Soc. Mag. Reson. Med. 2015;23:2486.
4. Saranathan M, Rettmann DW, Hargreaves BA, Clarke SE, Vasanawala SS. DIfferential Subsampling with Cartesian Ordering (DISCO): a High Spatio-temporal Resolution Dixon Imaging Sequence for Multiphasic Contrast Enhanced Abdominal Imaging. J Magn Reson Imaging 2012;35(6):1484-1492.
5. Huang C, Graff CG, Clarkson EW, Bilgin A, Altbach MI. T2 Mapping from Highly Undersampled Data by Reconstruction of Principal Component Coefficient Maps Using Compressed Sensing. Magn Reson Med 2012;67:1355–1336.
Figures