1386
Alteration of mechanical tissue parameters during progressive formalin fixation measured by broadband Magnetic Resonance Elastography using a compact and portable tabletop scanner1Department of Medical Informatics, Charité - Universitätsmedizin Berlin, Berlin, Germany, 2Department of Radiology, Charité - Universitätsmedizin Berlin, Berlin, Germany, 3Beuth Hochschule für Technik Berlin, Berlin, Germany, 4Pure Devices GmbH, Würzburg, Germany
Synopsis
A compact tabletop MR elastography (MRE) device was employed for rheological tests of soft tissue samples to measure the change of viscoelastic powerlaw constants in liver and brain tissue during progressive fixation. Shear-modulus dispersion functions were acquired from 300 to 5700Hz in animal tissues at different states of formaldehyde fixation and fitted by the rheological springpot-powerlaw model. Formalin fixation reduced viscosity and increased elasticity of liver tissue faster and to a higher degree than in brain tissue similar to the alteration of mechanical properties observed by in vivo elastography of hepatic fibrogenesis.
Purpose
Magnetic resonance elastography (MRE) shows a higher consistency of rheological constants of macroscopic soft biological tissue samples1 than surface based mechanical tests.2 We therefore developed a compact, automated tabletop MRE device and applied the new methods to test the change of viscoelastic powerlaw constants of tissue samples during progressive fixation. Fixation effects on tissue structures are highly relevant in pathology and transplantation medicine3 but have never been addressed by the springpot model – the most basic two-parameter powerlaw model in rheology.Methods
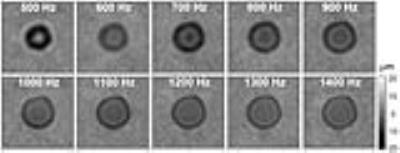
A tabletop MRI scanner (Pure Devices GmbH, Würzburg, Germany) with 0.5T permanent magnet was customized to MRE (Fig. 1) as described in a preliminary version in 4. Trapezoidal bipolar motion encoding gradients (MEG, amplitude=0.7T/m) centered around the refocusing pulse of a spin echo sequence were synchronized to the vibration frequency f. The number N of MEG-cycles varied from N=4 and N=78 for f=300 and 5700Hz, respectively. Further acquisition parameters: repetition time=300ms, echo time=32ms, slice thickness=2mm, matrix size=64×64, field of view=12.8mm, driving frequencies 300-4500 (500-5700)Hz for porcine brain (calf liver), eight wave dynamics, one average, total acquisition time for a single frequency including toggling of MEG polarity=5.2min. Fresh calf liver tissue and porcine brain tissue (brain stem) were cut in cylindrical cores (7.5mm diameter, 5 to 8mm length) were transferred into glass tubes for experiments. A total of 13 (14) samples of liver (brain) were immersed in formalin (4% formaldehyde in buffer solution) and measured at the following times (in hours): 0 (0, native sample), 1(1), 2(2), 3(3), 4(4), 5(5), 6(6), 7(7), 8(8), 10(10), 12(12), 14(14), 16(16), 18(18), 24(24), 26(26), 28(28), 36(36), 48(48), 55(50), 77(72). Due to the constrained axial motion, cylindrical shear waves emanated concentrically from the tissue border towards the center. Therefore, motion field acquisition was limited to the component perpendicular to the transverse image orientation. Postprocessing consisted of unwrapping phase difference data and Fourier transform in time. Resulting 2D-wave fields at driving frequencies f (Fig. 2) were fitted by the analytical solution of shear waves in a cylinder5,6 (Fig. 3). The calculated complex-valued wave number was translated into two real-valued quantities related to stiffness (shear wave speed c) and viscous attenuation (shear wave penetration rate a).7 To derive shear modulus-related parameters, c and a were directly fitted by the springpot model which predicts a monotonic increase of storage and loss modulus over increasing vibration frequencies by the parameters μ (shear modulus) and α (dimensionless powerlaw exponent).Results
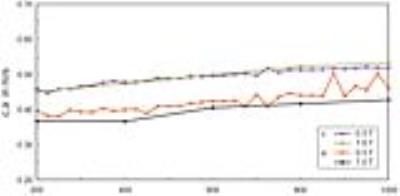
Reference measurements showed very good agreement with high field MRE performed on a preclinical scanner (Bruker PharmaScan 70/16, Ettlingen, Germany) using ultrasound gel of the same batch (Fig.4). Unfixated tissue of porcine brain stem was stiffer and higher dispersive than calf’s liver as reflected by μ=2.66kPa and α=0.66 versus μ=1.92kPa and α=0.42. Upon fixation, liver μ increased faster and reached higher values than brain μ, with decay times of approximately six versus ten hours and asymptotic shear moduli of 564±40 versus 134±25kPa. α in brain tissue decayed towards 0.39±0.02 in <1 hour and towards 0.23±0.01 in liver tissue in approximately 3 hours. Figure 5 shows springpot fit parameters μ and α versus the fixation time.Discussion
The harmonic stimulation of cylindrical waves allowed us to reduce the acquired information to single-slice, single-component encoding and analysis based on 2D-wave fitting. Unlike Laplacian-based reconstruction the model-fitting approach, (i) needs no filtering, (ii) shows no noise amplification, and (iii) is not affected by discretization artifacts up to the Nyquist limit. Thus, resolution limits and low SNR within short scan times can be alleviated making multifrequency MRE and viscoelastic dispersion measurements feasible for low-field MRI. Formalin as a crosslinker of proteins is known to increase tissue stiffness and reduce damping properties. This behavior was well reflected in the results obtained for tissue samples of porcine brain and calf liver. Brain tissue stiffening occurs less correlated with the reduction of loss properties compared to liver stiffening. For the latter formalin fixation seems to simulate the progressive change of viscoelastic tissue properties during hepatic fibrogenesis and might be a model of tissue mechanics and liver fibrosis.Conclusion
The progressive change of viscoelastic powerlaw constants of liver and brain tissue samples during fixation could be measured by a compact tabletop MRE device at 0.5T in automated fashion. The motion characteristics of the integrated piezoelectrical actuator allowed measurements in a range of mechanical frequencies from 300 to 5700Hz and supported filter-free fit-based cylindrical wave inversion. Formalin fixation changed liver tissue from highly viscous to elastic properties more than brain tissue.Acknowledgements
No acknowledgement found.References
1. Reiter R, Freise C, Jöhrens K, et al. Wideband MRE and static mechanical indentation of human liver specimen: Sensitivity of viscoelastic constants to the alteration of tissue structure in hepatic fibrosis. J Biomech. 2014;47:1665-1674.
2. Dittmann F, Hirsch S, Tzschaetzsch H, et al. In vivo wideband multifrequency MR elastography of the human brain and liver. Magn Reson Med. 2016;76:1116–1126.
3. Ayyildiz M, Aktas RG, Basdogan C. Effect of solution and post-mortem time on mechanical and histological properties of liver during cold preservation. Biorheology. 2014;51:47-70.
4. Samavati N, Körting C, Drießle T, et al. Compact and fully automated 3D multifrequency tabletop MR elastography for the measurement of viscoelastic parameters in small tissue samples. Proc ISMRM. 2016:4653.
5. Yasar TK, Royston TJ, Magin RL. Wideband MR elastography for viscoelasticity model identification. Magn Reson Med. 2013;70:479-489.
6. Okamoto RJ, Clayton EH, Bayly PV. Viscoelastic properties of soft gels: comparison of magnetic resonance elastography and dynamic shear testing in the shear wave regime. Phys Med Biol. 2011;56:6379-6400.
7. Tzschätzsch H, Guo J, Dittmann F, et al. Tomoelastography by multifrequency wave number recovery from time-harmonic propagating shear waves. Med Image Anal. 2016;30:1-10.
Figures