1380
Two novel low-cost 3D-printed mechanical actuators for MR elastography using exact end-to-end motion and centripetal force1Computer Assisted Clinical Medicine, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany
Synopsis
For reliable quantification of the shear modulus of soft tissues, MR elastography (MRE) needs consistent methods of low-frequency wave induction to the region of interest in the human body. This work proposes two novel designs of 3D-printed mechanical actuators powered by compressed air. Driver A offers constant and specific actuation amplitude independent of the chosen frequency of wave induction. Driver B employs centripetal force for wave induction. Contrary to conventionally used air cushions, the amplitude increases at higher frequencies, thus, making it suitable for high frequency MRE and multi-source wave induction.
Introduction
MR elastography (MRE) can serve as an additional diagnostic tool for the quantification of tissue stiffness, e.g. during prostate MR examination1 and may be used as a discriminator for benign prostatic hyperplasia or cancerous tissue.2 A major technical obstacle in MRE is the reliable wave induction in deep-lying tissues such as the prostate. Air cushions operate well at lower frequencies. However, the control of excitation amplitude becomes problematic at higher frequencies. Electromechanical actuators may distort images or need to be actively shielded.3 Furthermore, non-invasive techniques and a short additional set-up time to incorporate MRE in a routine prostate MR examination will facilitate clinical and patient acceptance in Europe.4 This work presents two novel low-cost 3D-printed mechanical actuators based on the principle of exact end-to-end motion of a mechanical extension and centripetal force for mechanical wave generation. Contrary to conventionally used air cushions, the amplitude increases at higher frequencies, thus, making it suitable for high frequency MRE and multi-source wave induction.Materials and Methods
Design of passive drivers:
Driver A converts rotational motion into translational motion (Fig.$$$\,$$$1A). A 3D-printed air turbine is powered by regulated compressed air available in all scanner rooms of the hospital. A shaft with a cam is connected to the turbine. The cam drives a translational motion (3$$$\,$$$mm amplitude) of a mechanical extension with two wave cycles per rotation. During MRE image acquisition, this driver is fixed on a mounting support.
Driver B is a 3D-printed pneumatic vibrator that creates centripetal force by an eccentric weight within the turbine (Fig.$$$\,$$$1B). The generated force ($$$F=m_{ecc}r_{ecc}\omega^2$$$) depends on the weight and center of mass of the eccentric weight as well as on the rotational speed of the turbine (Fig.$$$\,$$$3). Driver B fits in a pocket of the commercially available Body 18 coil (Siemens, Germany) for fixation and does not need additional mounting support.
All 3D-printed parts were designed with CAD software (Autodesk Inventor, Autodesk GmbH, Germany) and made of polyamide by selective laser sintering (Materialise GmbH, Germany).
Set up of control and feedback unit for active driver:
All MR-unsafe parts of the driver unit are placed in the control room. A proportional pressure regulator (Festo Vertrieb GmbH$$$\,$$$&$$$\,$$$Co. Kg, Germany) is connected to the in-house pressure hose and powers the air turbine of the passive drivers described above. The probe of an MR-safe fiber-optic sensor (Sick AG, Germany) provides feedback about the actuation frequency (Fig.$$$\,$$$2).
Image acquisition:
To evaluate both drivers, imaging on agarose-gelatin phantoms was performed on a 3T whole-body Magnetom Skyra (Siemens, Germany) using a four-channel receive only phase based-array coil. A commercially available gradient-echo based MRE sequence was employed (TE/TR$$$\,$$$=$$$\,$$$50/20$$$\,$$$ms, matrix$$$\,$$$=$$$\,$$$256x60, FOV$$$\,$$$=$$$\,$$$450$$$\,$$$mm, slice thickness$$$\,$$$=$$$\,$$$5$$$\,$$$mm).
Results and Discussion
The wave-induction frequencies of the passive drivers remained stable ($$$\pm$$$0.2$$$\,$$$Hz) during image acquisition as confirmed by a fiber-optic sensor feedback. The frequency can be regulated smoothly between 10 and 80$$$\,$$$Hz (driver A) and 10 and 130$$$\,$$$Hz (driver B). Both drivers do not produce artifacts in the acquired MR images (Fig.$$$\,$$$4) and are MR-safe since all magnetic and active electronic parts are placed outside the scanner room.
Driver A induces waves (Fig.$$$\,$$$4) with controlled amplitude that remains constant even at higher frequencies as the motion of the rod is coupled to cam and the end-to-end motion is always constant. Alternative waveforms and amplitudes can be designed with CAD software, 3D-printed and attached to the shaft. Its design enables wave-induction at locations that are difficult to reach using air cushions, e.g. for transperineal excitation during prostate MR examinations.
Driver B increases its wave-inducing force with increasing rotational speed and induces waves thoroughly in the phantom (Fig.$$$\,$$$4). Due to its compact design, it can be placed at various locations on the body and is easily set up and fixated using the body coil. Furthermore, more than one driver can be placed on the patient for multi-location wave-induction. Thus, driver B offers an elegant alternative to the commonly used air cushions where the amplitude of sound pressure waves dampens with increasing frequencies.
Conclusion
This work presents two mechanical actuators for MRE as an alternative to the conventionally applied air cushions. The driver is easy to set up and can be incorporated within existing equipment in the clinic. The passive drivers are MR–safe and neither interact with the gradients nor with the static magnetic field. Their design is adaptable and reproducible through low-cost 3D-printing. Future work will include volunteer studies in which varying locations and angles of surface wave induction will be evaluated.Acknowledgements
This research project is part of the Research Campus M²OLIE and funded by the German Federal Ministry of Education and Research (BMBF) within the Framework “Forschungscampus: public-private partnership for Innovations” under the funding code 13GW0092D.References
[1] R. Muthupillai, D. J. Lomas, P. J. Rossman, J. F. Greenleaf, A. Manduca, and R. L. Ehman, "Magnetic resonance elastography by direct visualization of propagating acoustic strain waves," Science, vol. 269, pp. 1854-7, Sep 29 1995.
[2] R. S. Sahebjavaher, G. Nir, M. Honarvar, L. O. Gagnon, J. Ischia, E. C. Jones, et al., "MR elastography of prostate cancer: quantitative comparison with histopathology and repeatability of methods," NMR Biomed, vol. 28, pp. 124-39, Jan 2015.
[3] K. Uffmann and M. E. Ladd, "Actuation systems for MR elastography: design and applications," IEEE Eng Med Biol Mag, vol. 27, pp. 28-34, May-Jun 2008.
[4] L. Dickinson, H. U. Ahmed, C. Allen, J. O. Barentsz, B. Carey, J. J. Futterer, et al., "Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting," Eur Urol, vol. 59, pp. 477-94, Apr 2011.
Figures
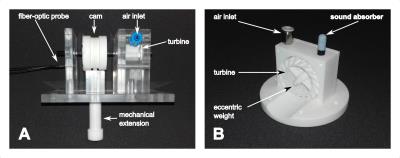
Fig. 1: Left: Photograph of passive driver A. Rotational motion is converted into translational motion using a shaft with an additional cam connected to the turbine. One revolution of the cam corresponds to two cosine wave cycles with peak-to-peak amplitude of 3 mm. Right: Photograph of passive driver B without side housing. Due to its compact design, it can be placed on various location of the body and it is possible to combine two or more drivers for multi-location wave induction.
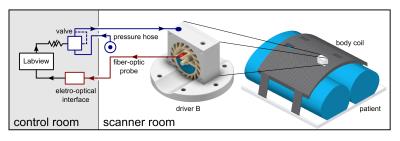
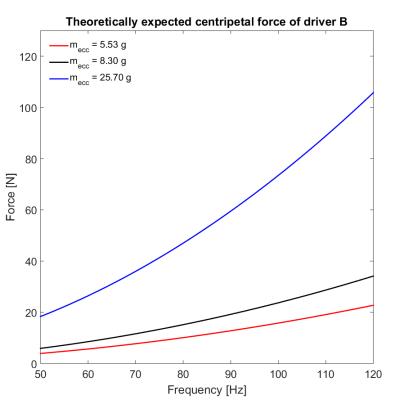

Fig. 4: Magnitude, phase and wave images of a gradient-echo based MRE sequence (top to bottom). Left: Wave induction with driver A in a homogeneous agarose phantom. Middle: Wave induction with driver A in a homogeneous agarose phantom. Right: Wave induction with driver B in a gelatin phantom with a cubical inclusion made of agarose yielding a higher elasticity compared to the gelatin background material.