1375
In vivo cerebral MR elastography in a mouse model of Alzheimer’s disease: preliminary results1Richard and Loan Hill Department of Bioengineering, University of Illinois at Chicago, Chicago, IL, United States
Synopsis
In vivo magnetic resonance elastography (MRE) experiments on Alzheimer’s disease (AD) mouse model were conducted. The AD and Control mice were in the age group of 3-4 months (n = 4 for both). Median stiffness values for the overall mouse brain (central axial slices) and for the hippocampus region were calculated. No differences were observed between the two groups for the overall brain. For the hippocampus region, a trend of cerebral stiffness decrease in AD was measured. The small sample size did not allow for statistically significant conclusions. Further experiments are underway.
Target Audience
Elastography, Alzheimer’s disease, and neurodegenerative disease diagnosis.Purpose
Alzheimer’s disease (AD) is the most widespread form of dementia, with an estimated 5.3 million in the United States alone suffering from the disease in 2015.1 AD is marked with the characteristic extracellular deposition of Amyloid-β (Aβ) plaques and decrease in brain tissue stiffness.2 Magnetic resonance elastography (MRE) has been demonstrated to be sensitive to the advanced stages of the disease3, but currently there is no clinical test for its early detection. The goal of the research is to show the early diagnostic potential of MRE for Alzheimer’s disease.Methods
Four APPswe/PS1dE9 female mice and four control mice were used for the study. The AD mice develop Aβ deposits in the hippocampus region of the brain.4 The mice used were in the age group of 3-4 months at the time of the experiments.
All measurements were performed using a 9.4T pre-clinical MRI scanner at the Research Resources Center of the University of Illinois at Chicago. In the elastography experiments, the mechanical shear waves were induced in the mouse brain through a vibrating bite bar type actuator. For measurement of the full 3D wave propagation, we used spin-echo based Sample Interval Modulation - magnetic resonance elastography (SLIM-MRE) which is capable of simultaneously encoding mono-frequency vibrations in all the three displacement directions.5
The scanner details and MRI/MRE imaging parameters are as follows: 38 mm volume radiofrequency (RF) coil, 16 mm2 field of view axial slices, 250 µm3 isotropic voxel size, TE/TR = 16.24/1000 ms, 3 averages, 1000 Hz actuation frequency, 250 mT/m motion encoding gradient (MEG) strength, 10 MEG cycles, and 8 time steps, with a total scan time of 51 min. 12 secs.
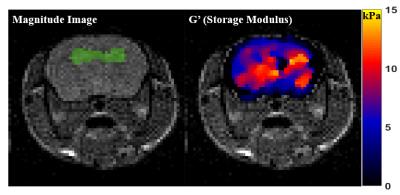
The curl operator was applied to the noise-filtered complex wave images and the complex shear modulus was calculated by the algebraic inversion of the Helmholtz equation.6 Median stiffness values or storage modulus (real part of the complex modulus) over 2 region of interests (ROIs) were calculated. ROI-1 is the overall area of the central brain axial slices as seen in figure 2. For ROI-2 (green highlighted region in figure 2), since Aβ-deposits first occur in the hippocampal region, part of the hippocampal area was manually segmented from MRI magnitude images, to check for differences in tissue stiffness.
Results
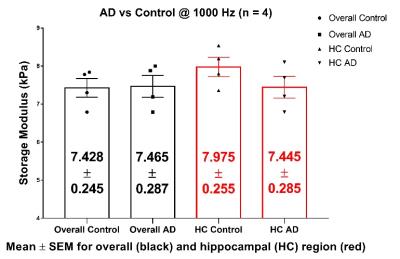
Median stiffness values (storage modulus, G’) for the 4 animals from both groups i.e. AD and Control were calculated and are shown in figure 3 as the plot points. We did not observe any differences in the shear stiffness averaged over ROI-1. Using ROI-2 (hippocampal area) we were able to measure a trend of stiffness decrease from the control and AD mice, but these differences were not statistically significant (p > 0.05).Discussion
There is a small, but not statistically significant difference in the brain tissue (hippocampus) stiffness between AD and control mice @ 3-4 months of age. We will conduct further experiments at later ages to examine the relation between Aβ deposition and brain stiffness. As of now, 3 AD mice at age 7-8 months have already been tested and the stiffness was decreased compared to the 3-4 month age group with 7.09 ± 0.22 kPa (mean ± standard error) storage modulus in the hippocampal region.Conclusion
In vivo cerebral SLIM-MRE of the mouse is feasible and may be useful to image the Aβ deposition in a mouse model of AD by means of hippocampal stiffness.Acknowledgements
Special thanks to Weiguo Li, Director of Research Service Facility (MRI), Research Resources Center, University of Illinois at Chicago, for his assistance with the MRI setup and experiments.References
1. Alzheimer’s, A. 2015 Alzheimer’s disease facts and figures. Alzheimer’s Dement. J. Alzheimer’s Assoc. 11, 332 (2015).
2. Murphy, M. C. et al. Decreased brain stiffness in Alzheimer’s disease determined by magnetic resonance elastography. J. Magn. Reson. Imaging 34, 494–498 (2011).
3. Murphy, M. C. et al. Magnetic resonance elastography of the brain in a mouse model of Alzheimer’s disease: initial results. Magn. Reson. Imaging 30, 535–9 (2012).
4. Jankowsky, J. L. et al. Mutant presenilins specifically elevate the levels of the 42 residue β-amyloid peptide in vivo: Evidence for augmentation of a 42-specific γ secretase. Human Molecular Genetics 13, 159–170 (2004).
5. Klatt, D., Yasar, T. K., Royston, T. J. & Magin, R. L. Sample interval modulation for the simultaneous acquisition of displacement vector data in magnetic resonance elastography: theory and application. Phys. Med. Biol. 58, 8663–75 (2013).
6. Manduca, A. et al. Magnetic resonance elastography: Non-invasive mapping of tissue elasticity. Med. Image Anal. 5, 237–254 (2001).
Figures