1371
Reproducibility Study of Direct and Non-Linear Inversion High-Resolution Magnetic Resonance Elastography (MRE) of the Hippocampus1Alzheimer Scotland Dementia Research Centre, University of Edinburgh, Edinburgh, United Kingdom, 2Clinical Research Imaging Centre, University of Edinburgh, Edinburgh, United Kingdom, 3Department of Biomedical Engineering, University of Delaware, 4Thayer School of Engineering, Dartmouth College, 5Department of Biomedical Engineering, Columbia University, 6Institute for Medical Informatics, Charité Universitätsmedizin Berlin, 7Department of Radiology, Mayo Clinic
Synopsis
Certain neurological disorders may not be detected with current clinical imaging modalities. Magnetic Resonance Elastography (MRE) combines acoustics with MRI to provide maps of tissue mechanical properties and may be sensitive to subtle tissue pathologies. Two published approaches for performing high-resolution MRE (so-called Direct Inversion and Non-Linear inversion) were applied to enable comparison of test-retest reproducibility of the hippocampus. Intraclass correlation coefficient found DI and NLI to display fair (0.42) and excellent (0.95) reproducibility, respectively, for measuring the magnitude of the complex shear modulus |G*|. Future work will assess the relative magnitude of technical and biological variance including both sex and ageing effects.
Introduction
Tissue mechanical properties can vary over several orders of magnitude in the disease state and can elude current clinical neuroimaging modalities. Magnetic Resonance Elastography (MRE) combines conventional MRI with acoustic wave propagation1 to generate high-resolution viscoelastic or ‘stiffness’ maps, and shows promise for the early detection and differential diagnosis of a wide-range of neurological disorders2-5. The purpose of this study was to evaluate the reproducibility of two published alternative approaches to performing high-resolution MRE of the hippocampus, a brain structure specifically implicated in Alzheimer’s disease (AD).
Methods
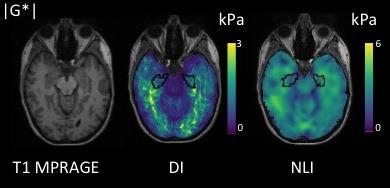
Six subjects (mean age 23.8 ± 3.5), underwent two MRE scans within 2-3 weeks. All data were acquired on a 3T Verio MRI system (Siemens Medical Systems, Erlangen, Grermany), with acoustic vibrations generated by the Resoundant actuator and head pillow (http://resoundant.com/). The DI protocol consisted of the acquisition of multiple frequency data (20, 30, 40, 50, 60Hz) using a modified Cartesian Echo Planar Imaging (EPI) sequence. The Elastography Software Pipeline (ESP)6 which incorporates algorithms for super-resolution Multi-frequency Elasto-Visco inversion (MDEV)7 was used to produce maps of viscoelastic parameters, namely the magnitude of the complex shear modulus |G*| and the phase angle φ, with an isotropic spatial resolution of 0.56 mm. The NLI protocol utilized a high-resolution spiral EPI acquisition8 at 50Hz with a Finite-Element-Model (FEM) based approach9 to measure the same parameters |G*| and φ at a 1.6mm spatial resolution. Freesurfer software10 was used to automatically segment the hippocampus on corresponding T1-weighted image 3D MR images, to which the elastograms had been co-registered13. The reproducibility of |G*| and φ for both protocols was determined using a two-way intra-class correlation coefficient (ICC) with the mean coefficient of variation (CV) also computed.Results
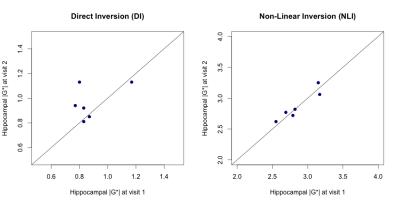
Example MRE images of DI and NLI are provided in Figure 1. A paired sample t-test found mean hippocampal |G*| to different significantly according to the analysis approach (DI: 0.92kPa ± 0.14kPa; NLI: 2.87kPa ± 0.23kPa, p < .001). Scatterplots of measurements at visit 1 versus visit 2 were evaluated (Figure 2). Mean |G*| of the hippocampus using DI was 0.96kPa ± 0.14kPa at visit 1, and 0.88kPa ± 0.15kPa at visit 2. Reproducibility of |G*| using DI was fair (0.42), with [ICC r (rxx)] ranging between -0.42 and 0.89, with a mean CV of 15.82 %. For NLI mean |G*| was 2.89 kPa ± 0.24kPa at visit 1, and 2.86 kPa ± 0.25 kPa at visit 2. Reproducibility of |G*| using NLI was excellent (0.95), with [ICC r (rxx)] ranging between 0.71 and 0.99, with CV of 8.55 %. For the phase angle φ, reproducibility was determined to be fair, with values of 0.50 and 0.52 for DI and NLI, respectively.
Discussion
High reproducibility of high-resolution MRE is essential in order to reliably detect changes in brain tissue that may occur as a result of neurological disease and motivate the adoption of MRE as a clinical tool. Two high-resolution brain MRE protocols were applied at two separate time points. NLI was more reproducible than DI for measuring |G*|. No discernible differences were found in the reproducibility of φ according to the analysis approach. The DI protocol, which utilises an EPI sequence for the fast acquisition of multi-frequency data, may benefit from correcting for susceptibility-induced image distortion12 to aid in the accuracy of the anatomical T1 and MRE co-registration and improve resolution. Incorporating soft-prior regularisation within the NLI analysis13, to promote local homogeneity in pre-defined regions, may minimize the contribution of other subcortical grey matter structures and CSF to further improve the reliability of the NLI based protocol14.
Conclusion
Brain MRE holds promise to be a reliable imaging technique for investigating the mechanical properties of specific brain structures in both health and disease.Acknowledgements
Lucy Hiscox is funded through a grant to the University of Edinburgh from Alzheimer Scotland. Prof Neil Roberts and Prof Edwin JR van Beek are supported by the Scottish Imaging Network, a Platform of Scientific Excellence (SINAPSE, www.sinapse.ac.uk ).References
1. Muthupillai R, Lomas D J, Rossman P J, Greenleaf J F, Manduca A and Ehman R L 1995. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves, Science, 269 1854–7.
2. Fattahi N, Arani A, Perry A, Meyer F, Manduca A, Glaser K, Senjem M L, Ehman R L and Huston J 2015. MR elastography demonstrates increased brain stiffness in normal pressure hydrocephalus AJNR Am. J. Neuroradiol. 37 462–7.
3. Huston J, Murphy M C, Boeve B F, Fattahi N, Arani A, Glaser K J, Manduca A, Jones D T and Ehman R L 2015. Magnetic resonance elastography of frontotemporal dementia J. Magn. Reson. Imaging 43 474–8.
4. Lipp A, Trbojevic R, Paul F, Fehlner A, Hirsch S, Scheel M, Noack C, Braun J and Sack I 2013. Cerebral magnetic resonance elastography in supranuclear palsy and idiopathic Parkinson’s disease Neuroimage Clin. 3 381–7.
5. Murphy M C, Jones D T, Jack C R, Glaser K J, Senjem M L, Manduca A, Felmlee J P, Carter R E, Ehman R L and Huston J 2016. Regional brain stiffness changes across the Alzheimer’s disease spectrum NeuroImage: Clin. 10 283–90.
6. Barnhill E Hollis L Sack I, Braun J, Hoskins P R, Pankaj P, Brown C, van Beek, E J and Roberts N 2016. Nonlinear Multiscale Regularisation in MR Elastography: Towards Fine Feature Mapping. Medical Image Analysis.
7. Papazoglou S et al. 2012 Phys. Med. Biol. 57 2329–2346.
8. Johnson C L, McGarry M D J, Van Houten E E W, Weaver J B, Paulsen K D, Sutton B P and Georgiadis J G 2013b Magnetic resonance elastography of the brain using multishot spiral readouts with selfnavigated motion correction Magn. Reson. Med. 70 404–12.
9. McGarry M D J, Van Houten E E W, Johnson C L, Georgiadis J G, Sutton B P, Weaver J B and Paulsen K D 2012 Multiresolution MR elastography using nonlinear inversion Med. Phys. 39 6388–96.
10. Fischl, B 2012. FreeSurfer. Neuroimage, 62 774-781.
11. Jenkinson M, Beckmann C F, Behrens T E, Woolrich M W, & Smith S M 2012. Fsl, Neuroimage, 62, 782-790.
12. Fehlner A, Hirsch S, Weygandt M, Christophel T, Barnhill E, Kadobianskyi M, Braun J, Bernarding J, Lützkendorf R, Sack I and Hetzer S 2016. Increasing the spatial resolution and sensitivity of magnetic resonance elastography by correcting for subject motion and susceptibility-induced image distortions. J. Magn. Reson. Imaging. doi:10.1002/jmri.25516
13. McGarry M, Johnson, C L, Sutton B P, Van Houten E E W, Georgiadis J G, Weaver J B, & Paulsen K D (2013). Including Spatial Information in Nonlinear Inversion MR Elastography Using Soft Prior Regularization. IEEE Transactions on Medical Imaging. 32 1901–1909.
14. Johnson C L, Schwarb H, McGarry M D J, Anderson A T, Huesmann G R, Sutton B P and Cohen N J 2016 Viscoelasticity of subcortical gray matter structures Hum. Brain Mapp.