1367
High Resolution 3D GRE Brain MR Elastography is Feasible with a High Performance Compact 3T ScannerArvin Arani1, Roger C Grimm1, Joshua D Trzasko1, Paul T Weavers1, Brandon J Nelson1, Richard Ehman1, Clifford R Jack1, Matthew A Bernstein1, and John Huston III1
1Radiology, Mayo Clinic, Rochester, MN, United States
Synopsis
Several groups have investigated the role of magnetic resonance elastography (MRE) for the diagnosis of neurological diseases, which has primarily been done using 2D imaging acquisitions. The objective of this study is to determine if 3D FGRE MRE can localize small regions of elevated stiffness for brain MRE applications on a high performance compact 3T head only scanner. This study demonstrated that a compact 3T scanner has sufficient gradient performance to successfully acquire high resolution 3D FGRE MRE exams, and localize inclusions as small as 1.75cm in diameter, which is not possible on a conventional 3T scanner.
Purpose
Several groups have investigated the role of magnetic resonance elastography (MRE), a non-invasive quantitative stiffness imaging technique capable of measuring the viscoelastic properties of tissues in vivo, for the diagnosis of neurological diseases. MRE has shown significant changes in brain stiffness in brain tumors (1,2), normal pressure hydrocephalus (3,4), multiple sclerosis (5,6), and different types of dementia (7,8). Furthermore, groups have established baseline measurements of brain stiffness in healthy volunteer populations by investigating cross-sectional changes in brain viscoelasticity with respect to age and sex (9-11). A majority of this work has been performed using a 2D echo-planar or spiral pulse sequences in order to measure a 3-dimensional displacement field. Two-dimensional acquisitions are limited by slice-to-slice phase variation, which can lead to artifacts and potential underestimation of tissue stiffness. Recently a 3D GRE (Gradient Recalled Echo) elastography pulse sequence has been developed to overcome these limitations. However, translating this into patient studies has not been possible due to low displacement sensitivity arising from gradient performance limitations on whole body MRI scanners(12). A novel C3T MRI scanner with gradients simultaneously capable of 80mT/m amplitude and 700 T/m/s slew rate on each axis has recently been developed (13-15). The objective of this study was to determine if 3D GRE could be performed on the C3T, localize small inclusions of elevated stiffness in a brain phantom, and on a patient with a vestibular Schwannoma.Methods
A volunteer and a patient (recruited under an IRB-approved protocol) were imaged with 3D GRE MRE after providing written informed consent. A 3D sagittal MRE was performed on a compact 3T scanner with the following parameters, 7.2 mT/m motion encoding gradients (MEG), mechanical vibration=60Hz, TE=20.4ms, TR=24.1ms, a 1 cycle 1-2-1 MEG pulse, motion encoding sensitivity (MENC) = 7.1 μm/rad, RBW = ±25kHz, FOV = 240x240x192mm3, acquisition matrix= 120x120x96, resolution = 2x2x2mm3, flip angle = 12, 2D ARC reduction=2(slice)x2(phase), 8 channel receiver array (Invivo, Gainesville Fl), scan time = 12:25. Realtime gradient pre-emphasis (16) and frequency shifting compensated additional concomitant fields from the asymmetric gradient design. The healthy volunteer was also scanned twice on a standard clinical 3T scanner (Signa HDxt, GE Healthcare) to compare displacement sensitivity between the two scanners. The first scan used the same imaging parameters as the C3T but with a maximum MEG amplitude of 40 mT/m, which resulted in a MENC = 14.3 μm/rad, with a scan time of 12:40 minutes. In order to try and match MENC values a second scan was done using a 2 cycle MEG pulse (MENC=7.1, scan time = 21:23 minutes). To evaluate the ability to resolve localized regions of stiffness, 3D GRE MRE was performed on the C3T using a polyvinyl chloride brain phantom with background stiffness of ~3.4 kPa and embedded spherical inclusions (~7.4 kPa stiffness) with diameters of 1.75cm, 2cm, 2.25cm, 2.5cm, 2.75cm, and 3cm (Figure 1). All acquisition parameters were the same as for the human subjects, but the vibration frequency was set to 80Hz in order to reduce standing wave effects and phase wrapping artifacts which were observed even when using the minimum possible driving amplitudes at 60Hz.Results
The shear wave displacement sensitivity difference between the C3T and the conventional 3T scanner in the healthy volunteer is shown in Figure 2. The C3T scanner produces the SNR and motion sensitivity necessary to capture the shear wave amplitudes, but the conventional, whole-body, 3T scanner does not. 3D GRE MRE elastograms overlaid on T1-wieghted images in a patient with a vestibular Schwannoma is shown in Figure 3. This demonstrates the feasibility of using the C3T head-only scanner for applications in localizing stiffness within and around a tumor. All inclusions in the brain phantom could be visualized and discerned from the background gel (Figure 4). This demonstrates the potential for high resolution 3D GRE exams to localize small regions of interest.Discussion and Conclusions
This study demonstrated that a C3T scanner has sufficient gradient performance to successfully perform high resolution 3D GRE MRE, which is not possible on conventional 3T scanners due to limitations like peripheral nerve stimulation(15). 3D GRE MRE was able to delineate the heterogeneous nature of a tumor including stiff and cystic regions in a tumor, and localize inclusions as small as 1.75cm in diameter in a phantom.Acknowledgements
This research was supported by National Institutes of Health R01 grants EB010065 and EB001981 (R.L.E.).References
1. Xu L, Lin Y, Han JC, Xi ZN, Shen H, Gao PY. Magnetic resonance elastography of brain tumors: preliminary results. Acta radiologica 2007;48(3):327-330. 2. Hughes JD, Fattahi N, Van Gompel J, Arani A, Meyer F, Lanzino G, Link MJ, Ehman R, Huston J. Higher-Resolution Magnetic Resonance Elastography in Meningiomas to Determine Intratumoral Consistency. Neurosurgery 2015;77(4):653-658. 3. Streitberger KJ, Wiener E, Hoffmann J, Freimann FB, Klatt D, Braun J, Lin K, McLaughlin J, Sprung C, Klingebiel R, Sack I. In vivo viscoelastic properties of the brain in normal pressure hydrocephalus. NMR in biomedicine 2011;24(4):385-392. 4. Fattahi N, Arani A, Perry A, Meyer F, Manduca A, Glaser K, Senjem ML, Ehman RL, Huston J. MR Elastography Demonstrates Increased Brain Stiffness in Normal Pressure Hydrocephalus. Am J Neuroradiol 2016;37(3):462-467. 5. Wuerfel J, Paul F, Beierbach B, Hamhaber U, Klatt D, Papazoglou S, Zipp F, Martus P, Braun J, Sack I. MR-elastography reveals degradation of tissue integrity in multiple sclerosis. NeuroImage 2010;49(3):2520-2525. 6. Streitberger KJ, Sack I, Krefting D, Pfuller C, Braun J, Paul F, Wuerfel J. Brain viscoelasticity alteration in chronic-progressive multiple sclerosis. PloS one 2012;7(1):e29888. 7. Murphy MC, Huston J, 3rd, Jack CR, Jr., Glaser KJ, Manduca A, Felmlee JP, Ehman RL. Decreased brain stiffness in Alzheimer's disease determined by magnetic resonance elastography. Journal of magnetic resonance imaging : JMRI 2011;34(3):494-498. 8. Huston J, Murphy MC, Boeve BF, Fattahi N, Arani A, Glaser KJ, Manduca A, Jones DT, Ehman RL. Magnetic Resonance Elastography of Frontotemporal Dementia. Journal of Magnetic Resonance Imaging 2016;43(2):474-478. 9. Arani A, Murphy MC, Glaser KJ, Manduca A, Lake DS, Kruse SA, Jack CR, Jr., Ehman RL, Huston J, 3rd. Measuring the effects of aging and sex on regional brain stiffness with MR elastography in healthy older adults. NeuroImage 2015;111:59-64. 10. Sack I, Beierbach B, Wuerfel J, Klatt D, Hamhaber U, Papazoglou S, Martus P, Braun J. The impact of aging and gender on brain viscoelasticity. NeuroImage 2009;46(3):652-657. 11. Sack I, Streitberger KJ, Krefting D, Paul F, Braun J. The influence of physiological aging and atrophy on brain viscoelastic properties in humans. PloS one 2011;6(9):e23451. 12. Grimm RC, Chen J, Ehman R. Reducing Time Samples Needed for MR Elastography. 2016; Singapore. ISMRM. 13. Weavers PT, Shu YH, Tao SZ, Huston J, Lee SK, Graziani D, Mathieu JB, Trzasko JD, Foo TKF, Bernstein MA. Technical Note: Compact three-tesla magnetic resonance imager with high-performance gradients passes ACR image quality and acoustic noise tests. Medical Physics 2016;43(3):1259-1264. 14. Foo T, Tan ET, Schenck J, Graziani D, Laskaris ET, Vermilyea M, Sabourin C, Shu Y, Huston J, Bernstein MA. Novel High Performance, Compact 3.0T MRI System for Imaging the Brain. 2016; Orlando, FLA. Military Health System Research Symposium (MHSRS). 15. Lee SK, Mathieu JB, Graziani D, Piel J, Budesheim E, Fiveland E, Hardy CJ, Tan ET, Amm B, Foo TK, Bernstein MA, Huston J, 3rd, Shu Y, Schenck JF. Peripheral nerve stimulation characteristics of an asymmetric head-only gradient coil compatible with a high-channel-count receiver array. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 2015. 16. Tao S, Weavers PT, Trzasko JD, Shu Y, Huston J, 3rd, Lee SK, Frigo LM, Bernstein MA. Gradient pre-emphasis to counteract first-order concomitant fields on asymmetric MRI gradient systems. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 2016.Figures

Figure 1: A) Photograph of 7.4 kPa polyvinyl chloride
spherical inclusions with diameters of 1.75cm, 2cm, 2.25cm, 2.5cm, 2.75cm, and
3cm. B) Embedded inclusions in 3.4 kPa background polyvinyl chloride. C) Fully
enclosed brain MRE phantom.
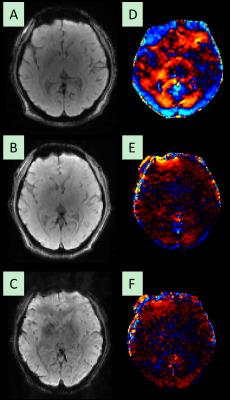
Figure 2: Wave displacement sensitivity comparison between the
compact 3T and a standard 3T scanner using a 2mm isotropic 3D GRE MRE pulse
sequence. A)-C) Magnitude images
obtained from the compact 3T, a motion encoding gradient time matched standard
3T scanner (10 minutes), and MENC matched (~20 minute scan duration) brain MRE
scan, respectively. D)-F) Corresponding
wave images demonstrating strong waves on the compact 3T D), absence of waves
on standard scanner with matched parameters E) and MENC matched acquisition F).
These results demonstrate that even doubling the scanning time to match motion
encoding sensitivity on a standard scanner does not resolve the displacement
amplitudes seen with the compact 3T.
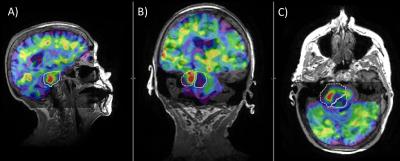
Figure
3: Sagittal (A), coronal (B), and axial (C) 3D GRE MRE elastograms overlaid on
T1-wieghted images in a patient with a vestibular Schwannoma (dashed outline)
with a posterior cystic (solid outline) region.
The MRE results show a stiff tumor core and a soft cystic region. This demonstrates the potential of the
compact 3T head-only scanner for applications in localizing stiffness within
and around a tumor.
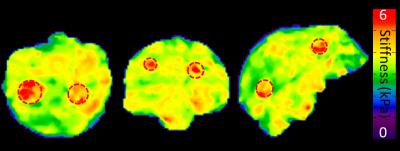
Figure 4: 3D elastograms in PVC brain phantom with embedded
stiffer inclusions (red dashed circles).
The diameter of the spheres in order from left to right are 3cm, 2.5cm,
1.75cm, 2.25cm, 2.75cm, 2cm.