1362
STEAM-CPMG: A method for localized Pulsed-Field-Gradient-Stimulated-Echo CPMG acquisitions1Institute of Myologie, La Pitié-Salpêtrière University Hospital, Paris, France, 2CEA/DRF/I2BM/MIRCen, Fontenay aux Roses, France, 3Institute for Diagnostic and Interventional Neuroradiology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland, 4Department of Neurology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
Synopsis
The CPMG method has been long applied for multi-component T2 studies which have been shown to reveal the micro-anatomical compartmentation of water in biological tissue. A new method for localized CPMG acquisitions that makes use of the Stimulated Echo Acquisition Mode technique is presented. Besides offering localized T2-relaxation data within less than 10 s, the method is suited for performing Diffusion-Relaxation-Correlation-Spectroscopy studies. Such studies allow evaluating the translational diffusion of the different T2-compartments observed in vivo and shall offer new insights on diffusion and relaxation processes in biological tissues. The method has been validated in vitro.
Purpose
NMRI
T2-mapping techniques represent important clinical tools by allowing the detection of
sites marked by alterations on tissue microstructure and water distribution,
which are usually characterized by elevated global water T2 values 1. Although such alterations usually reveal
scenarios of disease activity or traumatic injury, these may correspond to different
pathophysiological processes, such as interstitial oedema, necrosis, inflammation,
cell swelling, etc.. The Carr-Purcell-Meiboom-Gill (CPMG) sequence 2 allows the acquisition of T2-relaxation data
with echo-time sampling and SNR significantly higher than NMRI methods. Spatially localized CPMG acquisitions may be
accomplished by combining the CPMG with a Single-Voxel Selection method.
A recent in vivo multi-component T2 study 3 suggested that the multi-exponential behaviour
of the T2-relaxation in skeletal muscle reflects to some extent the anatomical
compartmentation into intracellular, interstitial and vascular spaces. The evaluation
of compartment-specific T2 alterations shall offer some specificity that is
missing in the current T2 maps 4 and contribute to the current understanding of
the relaxation processes observed in vivo. The present work describes the implementation
of a new method for localized CPMG acquisitions that makes use of the Stimulated
Echo Acquisition Mode (STEAM) technique 5. Besides offering localized T2-relaxation data
within less than 10 s of acquisition time, the proposed STEAM-CPMG method consists
on a modification of the Pulsed-Field-Gradient Stimulated-Echo CPMG
(PFGSTE-CPMG) sequence 6, thus opening the possibility for performing
Diffusion-Relaxation Correlation Spectroscopy (DRCOSY) studies 7 in vivo.Methods
Spatial localization was accomplished by replacing each of the first three hard pulses of the original PFGSTE-CPMG sequence (Fig1.a) by a spectrally selective Shinnar-Le Roux designed linear phase pulse. The CPMG echo train shall repeatedly refocus the localized stimulated echo (STE) produced by the STEAM module. Application of different diffusion gradient’ amplitudes in the TE/2 intervals (Fig1) and different TM intervals allows controlling the diffusion weighting of each T2-decay curve. In order to avoid phase coherences in spin ensembles other than the one producing the STE, the slice rephasing gradients were applied on the opposite side of the TM interval (Fig1.b), a spoiler gradient was applied during the TM interval and each acquisition was repeated once with the phase of the 1st selective RF-pulse shifted by 180°. Final data was obtained by subtraction of both acquisitions. PFGSTE-CPMG data were acquired from two 20 mL samples containing MnCl2 solutions at 0.15 and 0.30 mM. First, non-localized data were acquired by turning off the slice-selective gradients. Then, localized data were acquired from each sample by adjusting the STEAM voxel in order to encompass each phantom at a time. Both phantoms were placed inside the bird-cage transceiver coil during all three experiments. Sequence parameters were: TR = 5 s, TE = 36 ms, TM = 14 ms, IES = 2 ms and echo-train-length = 200, resulting in an acquisition time of 10 s per CPMG data. All CPMG acquisitions were repeated using nine different diffusion gradient amplitudes with corresponding b-values exponentially sampled between 9.818 s/mm2 and 981.8 s/mm2. 2D diffusion-relaxation (D-T2) spectra were extracted from each data set using 2D inverse Laplace transform 8. Experiments were performed in a 3T whole-body scanner (PRISMA, Siemens Healthcare, Erlangen, Germany).Results
The
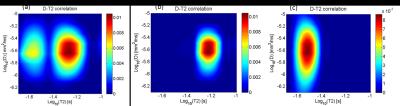
D-T2 spectra extracted from the non-localized data is presented in Fig2.a. The
distribution shows 2 distinct peaks centred at the T2-values around 27 and 59 ms,
both characterized by the same ADC around 2.5x10-6 mm2/ms.
The D-T2 distributions characterizing the solutions at 0.15 and 0.3 mM, extracted
from the localized acquisitions, are presented in Fig2.b and Fig2.c respectively.
As expected each distribution presents a single peak, both centred at the same ADC
(2.5x10-6 mm2/ms), while in the T2 direction the peaks
were centred around 59 ms and 29 ms for the solutions at 0.15 mM and 0.3 mM,
respectively.Conclusions
The results demonstrate that the localization works. As expected, the two peaks observed in the spectra characterizing the non-localized data (Fig 2.a) were separately observed in each spectrum characterizing the individual samples (Fig 2.b-c). The STEAM-CPMG opens the possibility for performing DRCOSY studies in vivo. More than reflecting intrinsic compartmental relaxation constants, the apparent T2-values observed in vivo are the result of complex processes of compartmental water exchange and magnetization transfer, which are directly related with molecular motion. Being able to distinguish between molecular interactions and molecular motion shall contribute to our current understanding of diffusion and relaxation processes in biological tissues, offering necessary information for increasing the accuracy of theoretical models for predicting the signal behaviour in NMRI methods, which has gained increasing importance in the current methodological development in NMR.Acknowledgements
No acknowledgement found.References
1. de Sousa P L et al. Magn Reson Med. 2012;67(5):1379-1390.
2. Meiboom S, Gill D. Rev Sci Instrum. 1958;29(8):688.
3. Araujo ECA, Fromes Y, Carlier PG. Biophys J. 2014;106(10):2267-2274.
4. Araujo ECA, Carlier PG. In: Proc. 23rd ISMRM, Toronto, Canada; (2015).
5. Frahm J, Merboldt K-D, Hänicke W. J Magn Reson. 1987;72(3):502-508.
6. Qiao Y, Galvosas P, Callaghan PT. Biophys J. 2005;89(4):2899-2905.
7. Godefroy S, Callaghan PT. Magn Reson Imaging. 2003;21(3-4):381-383.
8. Tønning E et al. J Magn Reson. 2007;188(1):10-23.
Figures