1361
Development of contrast agents for simultaneous PET/MRI of murine tumor models1University of Arizona, Tucson, AZ, United States, 2Pharmaceutical Sciences, University of Arizona, Tucson, AZ, United States, 3Medical Imaging, University of Arizona, Tucson, AZ, United States
Synopsis
PET/MRI contrast agents represent a new field of molecular imaging that provide an outstanding opportunity to employ simultaneous PET/MRI instrumentation for quantitative imaging. Over 90 responsive MRI contrast agents have been reported that change their contrast based on a biomarker and agent concentration, limiting their utility in vivo because a change in signal could arise from the presence of the biomarker and/or a change in the concentration of the agent. We propose to use PET to quantify the agent concentration, and a comparison of PET and MRI contrast can quantitatively evaluate the biomarker in a concentration-independent manner. Herein we describe the synthesis and characterization of 18F-radiolabeled responsive MRI contrast agents that are designed to measure pH and redox state using simultaneous PET/MRI for small animal studies.
Purpose
We sought to develop PET/MRI contrast agents that can quantitatively evaluate biomarkers such as pH and redox state. These agents consist of an 18F radioligand that is rapidly conjugated to a T1-relaxation or T2-relaxation-based MRI contrast agent. The radiolabel can be monitored with PET to measure the concentration of the agent. The MRI contrast agent changes image contrast based on the agent’s concentration and dependence on the biomarker. The simultaneous acquisition of PET and MRI images can be used to quantitatively measure the biomarker more specifically.Methods
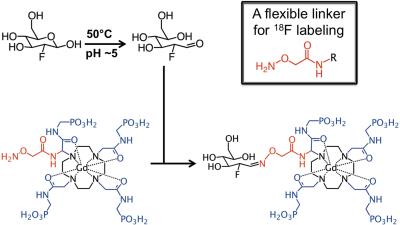
We have developed a synthesis method that conjugates an aminooxy ligand to the a-amino group of a Gd-DOTA derivative. 18F-fluorodeoxyglucose (18FDG) can selectively couple to an aminooxy group to form a stable oxime (Figure 1).1 We are using this method to couple 18FDG to Gd-DOTA-4AMP that can generate T1-weighted contrast that is dependent on pH and the concentration of the agent.2 The PET and MRI signals can be used to estimate the T1 relaxivity, and the pH can be calculated using a calibration curve between relaxivity and pH. Importantly, a 19F version of the agent can be synthesized using the same approach. A mixture of 18F- and 19F-labeled agent can be injected that have identical pharmacokinetics, so that 18F can be detected at ~10 nM concentration and the MRI contrast agent can be detected at ~100 mM concentration.
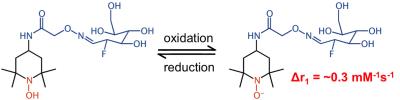
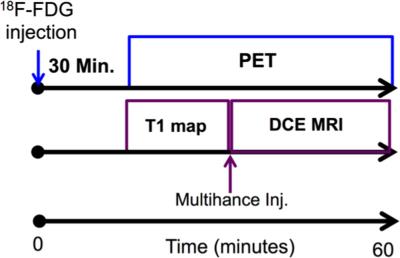
We have developed similar synthesis schemes that incorporate an aminooxy ligand in SLENU (Figure 2).3 The same radiolabeling of this MRI contrast agent with 18FDG leads to T1-weighted contrast that depends on redox status and agent concentration, and PET signal that is related to the agent concentration. A comparison of the simultaneous PET and MRI detection can lead to quantitative measurement of redox status. To simultaneously detect the PET signal and T1-weighted MRI contrast from our PET/MRI contrast agents, we have obtained a NuPETTM PET detector (Cubresa, Inc., Winnepeg MN) that inserts into a 7T magnet with a 20 cm bore of a Biospec MRI instrument (Bruker Biospin, Inc., Billerica, MA). To demonstrate simultaneous PET/MRI with contrast agents, we have simultaneously performed 18FDG PET imaging with Dynamic Contrast Enhanced (DCE) MRI, using the following protocols (Figures 3,4): PET Protocol, each mouse was injected with 1.50 mCi 18F-FDG 30 minutes before starting any imaging procedure; next, each mouse was anesthetized with 1.5% isoflurane and secured to a customized cradle manufactured by Cubresa, Inc. PET data was acquired in list mode continuously immediately after placing each mouse in the imaging scanner. A final single PET image was reconstructed using a back projection method and all of the acquired data. MRI Protocol: The following MRI scans were acquired for all mice: a) Anatomical localizer with an standard spin echo MRI protocol, b) Saturation recovery sequence used to estimate T1 time, c) T1-weighted MRI at a temporal resolution of 6.4 seconds. Next, Multihance was injected at 10 mM/Kg in 100 L of saline after ~60 seconds of initiation the DCE MRI scan and continued for 30 additional minutes. The MR images were analyzed with the Linear Reference Region Model.4
Results
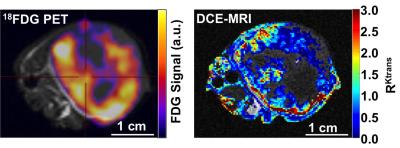
We have synthesized DOTA with the aminooxy ligand. We are currently refining our synthesis if DOTA-4AmP and SLENU with an aminooxy ligand (Figures 1,2). We are also refining methods to chelate Gd(III) and optimize the yield of coupling 19F-FDG and 18F-FDG to aminooxy-DOTA, aminooxy-DOTA-4AmP, and aminooxy-SLENU. The spatial distributions of the 18FDG uptake and the relative permeability of the tumor and muscle (RKtrans ) from DCE MRI were overlaid on an anatomical MR image (Figures 3,4). The results showed good 18FDG uptake and strong vascular permeability in the tumor rim relative to the core. Overall, these results demonstrated that simultaneous PET/MRI is feasible with PET and MRI contrast agents, and is therefore feasible with coupled PET-MRI contrast agents.Discussion
More generally, over 90 MRI contrast agents have been reported that are responsive to molecular biomarkers.5,6 Our research shows that many of these contrast agents can be easily modified to become PET/MRI contrast agents. In particular, MRI contrast agents that can measure oxygen saturation and can detect enzyme activity can be converted into PET/MRI agents. T2-relaxation MRI contrast agents can also be augmented to be PET/MRI contrast agents. A key development is the availability of simultaneous PET/MRI that can track these contrast agents, which provides an outstanding rationale for the continued development and dissemination of simultaneous PET/MRI instrumentation.Acknowledgements
No acknowledgement found.References
1. Namavari, M, et al. Bioconjugate Chem 2009, 20, 432-436.
2. Frullano L, et al. Angew. Chem. Int. Ed. 2010, 49, 2382-2384.
3. Zhelev Z, et al. Mol BioSyst 2012;8:2733-2740.
4. Cárdenas-Rodríguez J, et al. Magn Reson Imaging 2013;31(4):497-507.
5. Yoo B, et al. Front Biosci 2008;13:1733-1752.
6. Hingorani DV, et al. Contrast Media Molec Imaging 2015;10(4):245-265.